
CASODEX 150 mg FILM-COATED TABLETS

How to use CASODEX 150 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Casodex 150 mg Film-Coated Tablets
bicalutamide
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Casodex and what is it used for
- What you need to know before you take Casodex
- How to take Casodex
- Possible side effects
- Storage of Casodex
- Contents of the pack and other information
1. What is Casodex and what is it used for
Casodex belongs to a group of medicines called anti-androgens, which means it interferes with some of the actions of male sex hormones (androgens) in the body.
This medicine is used in adult men to treat prostate cancer that has not spread to other parts of the body, in cases where there is a high risk of the cancer spreading. It can be used alone or in combination with other treatments such as surgical removal of the prostate gland or radiotherapy.
2. What you need to know before you take Casodex
Do not take Casodex
- If you are allergic to bicalutamide or any of the other ingredients of this medicine (listed in section 6).
- If you are a woman.
- If you are taking terfenadine or astemizole, which are used to treat allergies, or cisapride, which is used to treat heartburn and acid reflux.
Casodex should not be given to children and adolescents under 18 years.
Warnings and precautions
Tell your doctor or pharmacist before you start taking Casodex:
- If you have any liver problems. The medicine should only be taken after your doctor has carefully considered the possible benefits and risks. If this is the case, your doctor will need to perform regular blood tests to check your liver function. Severe liver damage (changes in liver function and liver failure) has been reported.
- If you have a lung disease called interstitial lung disease. The symptoms may include severe difficulty breathing with cough or fever. Fatal cases have been reported.
- If you are taking any other medicines, including those you have bought without a prescription. In particular, tell your doctor if you are taking medicines that thin the blood or medicines to prevent blood clots.
- If you have severe kidney problems. Caution is needed as there is no experience with the use of bicalutamide in these cases.
- If you have heart problems. Your doctor may decide to check your heart function regularly.
Tell your doctor if you have any heart or blood vessel problems or are being treated for them, including medicines to control heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using Casodex 150 mg.
If any of the above applies to you, tell your doctor before taking Casodex.
If you are admitted to hospital, tell the medical staff that you are taking Casodex 150 mg.
If you are taking Casodex, you and/or your partner should use a contraceptive method while you are being treated with Casodex and for 130 days after finishing treatment.
Ask your doctor if you have any questions about contraceptive methods.
Children and adolescents
Casodex should not be given to children and adolescents under 18 years.
Other medicines and Casodex
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those you have bought without a prescription and herbal medicines.
Do not take bicalutamide with the following medicines:
- Terfenadine or astemizole (for hay fever or allergy).
- Cisapride (for stomach problems).
If you take Casodex with any of the following medicines, the effect of Casodex and the other medicine may be affected. Tell your doctor before taking any of the following medicines with bicalutamide:
- In particular, tell your doctor if you are taking blood-thinning medicines such as warfarin.
- Ciclosporin (used to suppress the immune system to prevent and treat organ or bone marrow transplant rejection).
- Midazolam (a medicine used to relieve anxiety before surgery or certain procedures or as an anaesthetic before and during surgery).
- Calcium channel blockers (e.g. diltiazem or verapamil. These medicines are used to treat high blood pressure or certain heart diseases).
- Cimetidine (for stomach ulcers).
- Ketoconazole (used to treat fungal infections of the skin and nails).
Casodex 150 mg may interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone and sotalol) or may increase the risk of heart rhythm problems when used with other medicines (e.g. methadone (used for pain relief and for detoxification from other medicines), moxifloxacin (an antibiotic), antipsychotics used to treat severe mental illnesses).
Please note that these warnings may also apply to medicines that you have used in the past.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking any medicine.
Casodex should not be taken by women, including pregnant or breast-feeding women.
Casodex may affect male fertility, which may be reversible.
Driving and using machines
Casodex may make you feel drowsy. Be careful when driving or using machines.
Casodex contains lactose
If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
Casodex contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per tablet, which is essentially 'sodium-free'.
3. How to take Casodex
Take this medicine exactly as your doctor has told you.
If you are not sure, ask your doctor or pharmacist.
- The recommended dose is one tablet a day.
- Swallow the tablet whole with a glass of water.
- Try to take the tablet at the same time each day.
If you take more Casodex than you should
If you have taken more than the prescribed dose, contact your doctor or the nearest hospital.
In case of overdose or accidental ingestion, contact the Toxicology Information Service. Telephone 91 562 04 20.
If you forget to take Casodex
Do not take a double dose to make up for a forgotten dose. Simply continue with the next dose as planned.
If you stop taking Casodex
Do not stop taking this medicine unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, tell your doctor immediately or go to the casualty department of your nearest hospital. These serious side effects are very rare.
- Skin rash, severe itching of the skin (with lumps), hives, skin peeling or blistering.
- Swelling of the face or neck, lips, tongue or throat, which may cause difficulty breathing or swallowing.
- Breathing difficulties or sudden worsening of breathing difficulties, possibly with cough or fever. These may be signs of interstitial lung disease.
- Blood in the urine.
- Abdominal pain.
- Yellowing of the skin and eyes (jaundice). These may be signs of liver damage.
Other possible side effects of this medicine include:
Very common: more than 1 in 10 patients
- Skin rash.
- Weakness.
- Breast enlargement and breast tenderness. Breast enlargement may not resolve spontaneously after stopping treatment, particularly after prolonged treatment.
Common: between 1 and 10 in 100 patients
- Weight gain.
- Anaemia.
- Dizziness, drowsiness.
- Abdominal pain, constipation, indigestion, flatulence (gas), nausea (feeling sick).
- Blood in the urine.
- Hair loss (alopecia), excessive hair growth/re-growth, dry skin, itching.
- Decreased appetite.
- Hot flushes.
- Chest pain, swelling.
- Liver toxicity, elevated liver enzymes, jaundice (yellowing of the skin and eyes).
- Impotence.
- Decreased libido.
- Depression.
Uncommon: between 1 and 10 in 1,000 patients
- Interstitial lung disease (inflammation of the lungs). Fatal cases have been reported.
- Allergic reactions (hypersensitivity), skin swelling, urticaria.
Rare: between 1 and 10 in 10,000 patients
- Liver failure. Fatal cases have been reported.
- Increased sensitivity to sunlight.
Cannot be estimated from the available data
- Changes in the electrocardiogram (ECG) (prolongation of the QT interval).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines and Healthcare Products Agency (AEMPS) website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Casodex
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Store in the original package.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Casodex contains
- The active substance is bicalutamide. Each tablet contains 150 mg of bicalutamide.
- The other ingredients are: Core; lactose monohydrate, potato starch, povidone (E1201), magnesium stearate (E470b). Coating; hypromellose (E464), macrogol 300 and titanium dioxide (E171).
Appearance and packaging
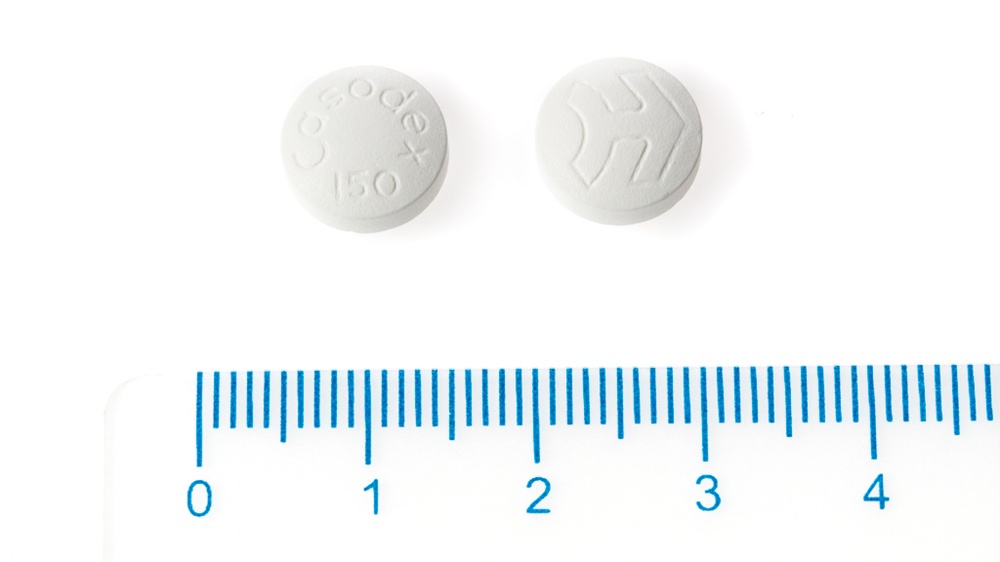
This medicine is available as film-coated tablets. The tablets are white, round, biconvex, engraved with the Casodex logo on one side and "Cdx 150" on the other.
Casodex 150 mg film-coated tablets are available in packs of 30 tablets in blisters.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Laboratoires Juvisé Pharmaceuticals
149 boulevard Bataille de Stalingrad
69100 Villeurbanne
France
Manufacturer
AstraZeneca AB Global External Sourcing (GES)
Astraallén
Gärtunaporten (B 674:5)
Södertälje, 151 85
Sweden
or
Corden Pharma GmbH
Otto-Hahn-Strasse
D-68723 Plankstadt
Germany
Date of last revision of this leaflet:June 2020
Detailed information on this medicine is available on the website of the Spanish Medicines and Healthcare Products Agency (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price127.09 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CASODEX 150 mg FILM-COATED TABLETSDosage form: TABLET, 50 mgActive substance: bicalutamideManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 50 mgActive substance: bicalutamideManufacturer: Almus Farmaceutica S.A.U.Prescription requiredDosage form: TABLET, 150 mgActive substance: bicalutamideManufacturer: Eugia Pharma (Malta) LimitedPrescription required
Online doctors for CASODEX 150 mg FILM-COATED TABLETS
Discuss questions about CASODEX 150 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













