CABAZITAXEL ZENTIVA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION

How to use CABAZITAXEL ZENTIVA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Cabazitaxel Zentiva 60 mg concentrate and solvent for solution for infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What Cabazitaxel Zentiva is and what it is used for
- What you need to know before you are given Cabazitaxel Zentiva
- How to use Cabazitaxel Zentiva
- Possible side effects
- Storage of Cabazitaxel Zentiva
- Contents of the pack and other information
1. What Cabazitaxel Zentiva is and what it is used for
Cabazitaxel Zentiva contains the active substance cabazitaxel, which belongs to a group of medicines called "taxanes", used to treat cancers.
Cabazitaxel is used to treat prostate cancer that has progressed after receiving other chemotherapy. It works by stopping the growth of cells and their multiplication.
As part of your treatment, you will also take a corticosteroid (prednisone or prednisolone) every day by mouth. Ask your doctor for information about this other medicine.
2. What you need to know before you are given Cabazitaxel Zentiva
Do not use Cabazitaxel Zentiva:
- if you are allergic (hypersensitive) to cabazitaxel, other taxanes, polysorbate 80, or any of the other ingredients of this medicine (listed in section 6),
- if your white blood cell count is very low (neutrophil count less than or equal to 1,500/mm3),
- if you have severe liver problems,
- if you have recently been or are going to be vaccinated against yellow fever.
Do not receive Cabazitaxel Zentiva if any of the above applies to you. If you are not sure, consult your doctor before receiving Cabazitaxel Zentiva.
Warnings and precautions
Before starting treatment with Cabazitaxel Zentiva, you will have blood tests to check that you have enough blood cells and that your kidneys and liver are working properly to receive Cabazitaxel Zentiva.
Tell your doctor immediately if:
- you have a fever. During treatment with Cabazitaxel Zentiva, it is more likely that your white blood cell count will decrease. Your doctor will monitor your blood and general condition to detect signs of infection. You may be given other medicines to maintain your blood cell count. People with low cell counts may develop life-threatening infections. The first sign of infection may be a fever, so if you have a fever, tell your doctor immediately.
- you have ever had any allergy. During treatment with Cabazitaxel Zentiva, severe allergic reactions may occur.
- you have severe or persistent diarrhea, feel unwell (nausea) or are being sick (vomiting). Any of these situations can cause severe dehydration. Your doctor should give you treatment.
- you have a feeling of numbness, tingling, burning, or decreased sensations in your hands and feet.
- you have any bleeding problems in your intestine or have changes in the color of your stools or stomach pain. If the bleeding or pain is severe, your doctor will interrupt your treatment with Cabazitaxel Zentiva. This is because Cabazitaxel Zentiva may increase the risk of bleeding or development of perforations in the intestinal wall.
- you have kidney problems.
- you have yellowing of the skin and eyes, dark urine, intense nausea (feeling of discomfort) or vomiting, as these may be signs or symptoms of liver problems.
- you notice that the volume of your urine increases or decreases significantly.
- you have blood in your urine.
If any of the above happens to you, tell your doctor immediately. Your doctor may reduce the dose of Cabazitaxel Zentiva or interrupt treatment.
Using Cabazitaxel Zentiva with other medicines
- Tell your doctor, pharmacist, or nurse if you are using or have recently used other medicines, including those obtained without a prescription. This is because some medicines may affect the effectiveness of Cabazitaxel Zentiva or Cabazitaxel Zentiva may affect the effectiveness of other medicines. These medicines include:
- ketoconazole, rifampicin (for infections);
- carbamazepine, phenobarbital, or phenytoin (for seizures);
- St. John's Wort or hypericum (a medicinal plant used to treat depression and other problems);
- statins (such as simvastatin, lovastatin, atorvastatin, rosuvastatin, or pravastatin) (to reduce cholesterol in your blood);
- valsartan (for hypertension);
- repaglinide (for diabetes).
While you are being treated with Cabazitaxel Zentiva, consult your doctor before getting vaccinated.
Pregnancy, breastfeeding, and fertility
Cabazitaxel Zentiva is not indicated for use in women.
Use condoms in your sexual relationships if your partner is or may be pregnant. Cabazitaxel may be present in your semen and affect the fetus. It is recommended not to father a child during and up to 4 months after treatment and to ask for information about sperm preservation before treatment, as Cabazitaxel Zentiva may alter male fertility.
Driving and using machines
During treatment with this medicine, you may feel tired or dizzy. If this happens, do not drive or use tools or machines until you feel better.
Cabazitaxel Zentiva contains ethanol (alcohol)
This medicine contains 709.8 mg of alcohol (ethanol) in each vial of solvent. The amount in the dose of this medicine is equivalent to 14 ml of beer or 6 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect. If you have alcohol addiction, have liver disease, or have epilepsy, consult your doctor or pharmacist before taking this medicine.
3. How to use Cabazitaxel Zentiva
Instructions for use
Before receiving cabazitaxel, you will be given anti-allergic medicines to reduce the risk of allergic reactions.
- Cabazitaxel will be administered by a doctor or nurse.
- Cabazitaxel must be prepared (diluted) before administration. This leaflet provides practical information for the handling and administration of cabazitaxel for doctors, nurses, and pharmacists.
- Cabazitaxel will be administered in the hospital through a drip (infusion) in one of your veins (intravenously) for approximately 1 hour.
- As part of your treatment, you will also take a corticosteroid medicine (prednisone or prednisolone) by mouth every day.
How much and how often it is administered
- The usual dose depends on your body surface area. Your doctor will calculate your body surface area in square meters (m2) and decide the dose you should receive.
- Usually, you will receive an infusion every 3 weeks.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Cabazitaxel Zentiva can cause side effects, although not everybody gets them. Your doctor will discuss this with you and explain the risks and potential benefits of your treatment.
Go to your doctor immediately if you notice any of the following side effects:
- fever (high temperature). This is common (may affect up to 1 in 10 people).
- severe loss of body fluids (dehydration). This is common (may affect up to 1 in 10 people). This can happen if you have severe or persistent diarrhea, or fever, or if you have been vomiting.
- severe stomach pain or stomach pain that does not go away. This can happen if you have a perforation in the stomach, esophagus, or intestine (gastrointestinal perforation). This can be life-threatening.
If any of the above happens to you, tell your doctor immediately.
Other side effects include:
Very common (may affect more than 1 in 10 people):
- reduction in the number of red blood cells (anemia), or white blood cells (which are important for fighting infections)
- reduction in the number of platelets (which results in an increased risk of bleeding)
- loss of appetite (anorexia)
- stomach upset, including nausea, vomiting, diarrhea, or constipation
- back pain
- blood in the urine
- fatigue, weakness, or lack of energy
Common (may affect up to 1 in 10 people):
- altered taste
- shortness of breath
- cough
- abdominal pain
- temporary hair loss (in most cases, hair grows back normally)
- joint pain
- urinary tract infection
- low white blood cell count associated with fever and infection
- feeling of numbness, tingling, burning, or decreased sensations in hands and feet
- dizziness
- headache
- increase or decrease in blood pressure
- stomach upset, heartburn, or belching
- stomach pain
- hemorrhoids
- muscle spasms
- frequent or painful urination
- urinary incontinence
- kidney problems
- mouth or lip ulcers
- infections or risk of infections
- high blood sugar levels
- insomnia
- confusion
- feeling of anxiety
- feeling of numbness or loss of sensation or pain in hands and feet
- balance problems
- rapid or irregular heartbeat
- blood clots in the legs or lungs
- feeling of suffocation in the skin
- mouth or throat pain
- rectal bleeding
- disorders, weakness, or pain in the muscles
- inflammation of the feet or legs
- chills.
- nail disorders (change in nail color; nails may come off).
Uncommon (may affect up to 1 in 100 people):
- low potassium levels in the blood
- ringing in the ears
- feeling of heat in the skin
- inflammation of the bladder, which can occur when your bladder has previously been exposed to radiation therapy (radiation recall cystitis).
Frequency not known (cannot be estimated from the available data):
- interstitial lung disease (inflammation of the lungs causing cough and difficulty breathing).
Reporting of side effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cabazitaxel Zentiva
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vials after EXP. The expiry date is the last day of the month stated.
Do not refrigerate.
In the section "Practical information for healthcare professionals on the preparation, administration, and handling of Cabazitaxel Zentiva" is included information on the storage and use time of Cabazitaxel Zentiva, once it has been diluted and is ready to use.
The disposal of unused medicine will be done according to local regulations. These measures will help protect the environment.
6. Contents of the pack and additional information
Composition of Cabazitaxel Zentiva
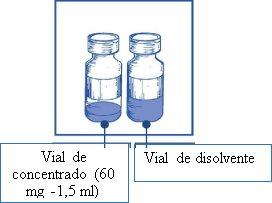
The active substance is cabazitaxel. One ml of concentrate contains 40 mg of cabazitaxel. One vial of concentrate contains 60 mg of cabazitaxel.
The other components are: polysorbate 80, citric acid, and absolute ethanol in the concentrate, and 96% ethanol and water for injectable preparations in the solvent (see section 2 "Cabazitaxel Zentiva contains ethanol (alcohol)").
Note: both the vial of Cabazitaxel Zentiva 60 mg/1.5 ml concentrate (fill volume: 1.83 ml) and the vial of solvent (fill volume: 5.67 ml) contain an overfill to compensate for liquid loss during preparation. This overfill ensures that after dilution with the complete content of the provided solvent, there is a solution containing 10 mg/ml of cabazitaxel for injection.
Appearance of the product and pack contents
Cabazitaxel Zentiva is a concentrate and solvent for solution for infusion (sterile concentrate).
The concentrate is a viscous, transparent solution between colorless and pale yellow.
The solvent is a transparent, colorless solution.
A pack of Cabazitaxel Zentiva contains:
Concentrate: 1.5 ml of concentrate in a 15 ml transparent tubular glass vial (type I), closed with a gray rubber elastomer stopper, sealed with a flip-off aluminum seal with a yellow plastic disc. Each vial contains 60 mg of cabazitaxel in 1.5 ml (nominal volume).
Solvent: 5.67 ml in a 15 ml transparent glass vial (type I), closed with a gray rubber elastomer stopper, sealed with a flip-off aluminum seal with a dark blue plastic disc. Each vial contains 5.67 ml (nominal volume).
Marketing authorization holder and manufacturer
Marketing authorization holder
Zentiva, k.s.,
U kabelovny 130,
Prague 10 – Dolní Mecholupy,
102 37 Czech Republic
Manufacturer
Mias Pharma Limited
Suite 2, Stafford House, Strand Road
Portmarnock, Co. Dublin
Ireland
Tillomed Malta Limited,
Malta Life Sciences
Park, LS2.01.06
Industrial Estate,
San Gwann, SGN 3000,
Malta
You canrequest more information about thismedicinal productby contacting the local representative of the marketing authorization holder:
Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Country | Product name |
Germany | Cabazitaxel Tillomed 60 mg concentrate and solvent for solution for infusion |
Spain | Cabazitaxel Zentiva 60 mg concentrate and solvent for solution for infusion EFG |
Italy | Cabazitaxel Tillomed |
France | Cabazitaxel Tillomed 60 mg solution to be diluted and solvent for solution for infusion |
Date of the last revision of this leaflet:July 2023
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
<------------------------------------------------------------------------------------------------------>
The following information is intended only for healthcare professionals.
PRACTICAL INFORMATION FOR DOCTORS OR HEALTHCARE PROFESSIONALS ON THE PREPARATION, ADMINISTRATION, AND HANDLING OF Cabazitaxel Zentiva 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
This information complements sections 3 and 5 for the user.
It is essential that you read the entire content of this procedure before preparing the infusion solution.
Incompatibilities
This medicinal product must not be mixed with other medicinal products except those used for dilutions.
Shelf life and special precautions for storage
For the pack of Cabazitaxel Zentiva 60 mg concentrate and solvent
Do not refrigerate.
After opening the vial
The vials of concentrate and solvent must be used immediately. If not used immediately, the time and storage conditions are the responsibility of the user. From a microbiological point of view, the two-stage dilution process must be carried out under controlled and aseptic conditions (see "Precautions for preparation and administration" below).
After the initial dilutionof Cabazitaxel Zentiva 60 mg concentrate with the completecontent of the solvent vial, chemical and physical stability has been demonstrated for 1 hour at room temperature (15°C - 30°C).
After the final dilution in the infusion bag/bottle
Chemical and physical stability of the infusion solution has been demonstrated for 8 hours at room temperature (15°C – 30°C), including 1 hour of infusion time, and for 48 hours in the refrigerator, including 1 hour of infusion time.
From a microbiological point of view, the infusion solution must be used immediately. If not used immediately, the storage times and conditions are the responsibility of the user and should not normally exceed 24 hours at 2°C - 8°C, unless the dilution has been carried out under controlled and validated aseptic conditions.
Precautions for preparation and administration
As with other antineoplastic agents, caution should be exercised during the preparation and administration of cabazitaxel solutions, taking into account the use of safety devices, personal protective equipment (e.g., gloves), and preparation procedures.
If, at any stage of preparation, cabazitaxel comes into contact with the skin, wash immediately and thoroughly with water and soap. If it comes into contact with mucous membranes, wash immediately and thoroughly with water.
Cabazitaxel should only be prepared and administered by trained personnel in the handling of cytotoxic agents. Pregnant healthcare workers should not handle it.
Always dilute the concentrate for solution for infusion with the complete solvent provided before adding it to the infusion solutions.
Preparation stages
Read this entire section carefully before mixing and diluting. Cabazitaxel Zentiva requires TWOdilutions before administration. Follow the preparation instructions provided below.
Note: both the vial of Cabazitaxel Zentiva 60 mg/1.5 ml concentrate (fill volume: 1.83 ml) and the vial of solvent (fill volume: 5.67 ml) contain an overfill to compensate for liquid loss during preparation. This overfill ensures that after dilution with the COMPLETEcontent of the provided solvent, there is a solution containing 10 mg/ml of cabazitaxel.
To prepare the infusion solution, the following two-stage dilution process must be carried out aseptically.
Stage 1: Initial dilution of the concentrate for solution for infusion with the provided solvent.
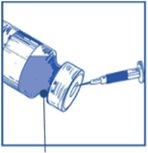
Stage 1.1 Inspect the vial of concentrate and the provided solvent. The concentrate and solvent solutions must be transparent |
|
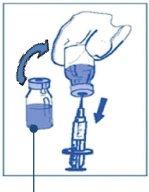
Stage 1.2 Using a syringe with a fixed needle, aseptically withdraw the completecontent of the provided solvent by partially inverting the vial. |
|
| |
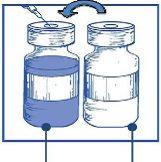
Stage 1.3 Inject the completecontent into the corresponding vial of concentrate. To limit foam formation as much as possible when injecting the solvent, direct the needle towards the inner wall of the concentrate vial and inject slowly. Once reconstituted, the resulting solution contains 10 mg/ml of cabazitaxel. |
|
Stage 1.4 Remove the syringe and needle, and manually mix gently by repeated inversions until a transparent and homogeneous solution is obtained. This may take about 45 seconds. |
|
| |
Stage 1.5 Let the solution stand for approximately 5 minutes, then check that the solution is homogeneous and transparent. It is normal for foam to persist after this time. |
|
This resulting concentrate-solvent mixture contains 10 mg/ml of cabazitaxel (at least 6 ml of released volume). The second dilution must be carried out immediately (before 1 hour) as detailed in Stage 2.
More than one vial of concentrate-solvent mixture may be necessary to administer the prescribed dose.
Stage 2: Final dilution (for infusion)
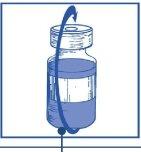
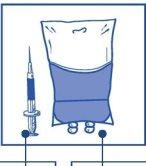
Stage 2.1 Aseptically withdraw the required amount of concentrate-solvent mixture (10 mg/ml of cabazitaxel) using a graduated syringe with a fixed needle. For example, a dose of 45 mg of Cabazitaxel Zentiva would require 4.5 ml of the concentrate-solvent mixture prepared in Stage 1. As there may still be foam on the wall of the vial of this solution, after the preparation described in Stage 1, it is preferable to place the syringe needle in the middle of the content during withdrawal. |
|
Stage 2.2 Inject into a sterile, non-PVC infusion bag or bottle of 5% glucose solution or 9 mg/ml (0.9%) sodium chloride solution for infusion. The concentration of the infusion solution must be between 0.10 mg/ml and 0.26 mg/ml. |
|
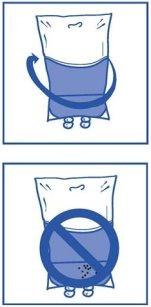
Stage 2.3 Remove the syringe and mix the contents of the infusion bag or bottle manually by rocking movement. Stage 2.4 As with all parenteral products, the resulting infusion solution must be visually inspected before use. Since the infusion solution is supersaturated, it may crystallize over time. In this case, the solution must not be used and must be discarded. |
|
The infusion solution must be used immediately. However, the in-use storage time may be longer under the specific conditions mentioned in the section Shelf life and special precautions for storage.
Disposal of unused medicinal products and all materials that have come into contact with them will be carried out in accordance with local regulations.
Method of administration
Cabazitaxel Zentiva is administered by infusion over 1 hour.
The use of an in-line filter with a 0.22 micrometer nominal pore size (also known as 0.2 micrometers) is recommended during administration.
PVC infusion bags or polyurethane infusion sets must not be used for the preparation and administration of the infusion solution.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CABAZITAXEL ZENTIVA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 20 mg/mlActive substance: cabazitaxelManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE INFUSION, 20 mgActive substance: cabazitaxelManufacturer: Eugia Pharma (Malta) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 60 mgActive substance: cabazitaxelManufacturer: Reddy Pharma Iberia S.A.Prescription required
Online doctors for CABAZITAXEL ZENTIVA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
Discuss questions about CABAZITAXEL ZENTIVA 60 mg CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions