
CABAZITAXEL AUROVIT 20 mg/mL concentrate for infusion solution

How to use CABAZITAXEL AUROVIT 20 mg/mL concentrate for infusion solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Cabazitaxel Aurovit 20 mg/ml Concentrate for Solution for Infusion
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Cabazitaxel Aurovit and what is it used for
- What you need to know before you are given Cabazitaxel Aurovit
- How to use Cabazitaxel Aurovit
- Possible side effects
- Storage of Cabazitaxel Aurovit
- Contents of the pack and other information
1. What is Cabazitaxel Aurovit and what is it used for
The name of your medicine is Cabazitaxel Aurovit. Its common name is cabazitaxel. It belongs to a group of medicines called “taxanes”, used to treat cancers.
Cabazitaxel is used to treat prostate cancer that has progressed after receiving other chemotherapy. It works by stopping the growth of cells and their multiplication.
As part of your treatment, you will also take a corticosteroid (prednisone or prednisolone) every day by mouth. Ask your doctor for information about this other medicine.
2. What you need to know before you are given Cabazitaxel Aurovit
Do not useCabazitaxel Aurovit
- if you are allergic to cabazitaxel, other taxanes, polysorbate 80, or any of the other ingredients of this medicine (listed in section 6).
- if your white blood cell count is very low (neutrophil count less than or equal to 1,500/mm3).
- if you have severe liver problems.
- if you have recently been or are going to be vaccinated against yellow fever.
You should not receive cabazitaxel if any of the above circumstances apply to you. If you are not sure, consult your doctor before receiving this medicine.
Warnings and precautions
Before starting treatment with this medicine, you will have blood tests to check that you have enough blood cells and that your kidneys and liver are working properly to receive cabazitaxel.
Tell your doctor immediately if:
- you have a fever. During treatment with cabazitaxel, it is more likely that your white blood cell count will decrease. Your doctor will check your blood and general condition to detect signs of infection. You may be given other medicines to keep your blood cell count up. People with low cell counts can develop life-threatening infections. The first sign of infection may be a fever, so if you have a fever, tell your doctor immediately.
- you have ever had any allergy. During treatment with this medicine, serious allergic reactions can occur.
- you have severe or persistent diarrhea, feel unwell (nausea) or are being sick (vomiting). Any of these situations can cause severe dehydration. Your doctor should give you treatment.
- you have a feeling of numbness, tingling, burning, or decreased sensation in your hands and feet.
- you have any bleeding problems in the intestine or have changes in the color of your stools or stomach pain. If the bleeding or pain is severe, your doctor will interrupt your treatment with cabazitaxel. This is because this medicine may increase the risk of bleeding or development of perforations in the intestinal wall.
- you have kidney problems.
- you have yellowing of the skin and eyes, dark urine, severe nausea (feeling unwell) or vomiting, as these may be signs or symptoms of liver problems.
- you notice that the volume of your urine increases or decreases significantly.
- you have blood in your urine.
If any of the above circumstances apply to you, tell your doctor immediately. Your doctor may reduce the dose of this medicine or interrupt treatment.
Other medicines and Cabazitaxel Aurovit
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription. This is because some medicines can affect the effectiveness of cabazitaxel or this medicine may affect the effectiveness of other medicines. These medicines include:
- ketoconazole, rifampicin (for infections).
- carbamazepine, phenobarbital, or phenytoin (for seizures).
- St. John's Wort or Hypericum (Hypericum perforatum) (a herbal medicine used to treat depression and other problems).
- statins (such as simvastatin, lovastatin, atorvastatin, rosuvastatin, or pravastatin) (to reduce cholesterol in your blood).
- valsartan (for high blood pressure).
- repaglinide (for diabetes).
While you are being treated with cabazitaxel, consult your doctor before being vaccinated.
Pregnancy, breastfeeding, and fertility
This medicine is not indicated for use in women.
Use condoms during sexual intercourse if your partner is or may become pregnant. Cabazitaxel may be present in your semen and may affect the fetus. It is recommended not to father a child during and up to 4 months after treatment and to seek information about sperm preservation before treatment, as this medicine may alter male fertility.
Driving and using machines
During treatment with this medicine, you may feel tired or dizzy. If this happens, do not drive or use tools or machines until you feel better.
Cabazitaxel Aurovit contains ethanol (alcohol)
This medicine contains 1.185 mg of alcohol (ethanol) per vial, which is equivalent to 395 mg/ml. The amount in each vial of this medicine is equivalent to 30 ml of beer or 12 ml of wine.
It is unlikely that the amount of alcohol in this medicine will have any noticeable effect on adults or adolescents. It may have some effects on young children, such as drowsiness.
The amount of alcohol in this medicine may alter the effect of other medicines. Consult your doctor or pharmacist if you are taking other medicines.
If you are pregnant or breastfeeding, consult your doctor or pharmacist before taking this medicine.
If you have an alcohol addiction, consult your doctor or pharmacist before taking this medicine.
3. How to use Cabazitaxel Aurovit
Instructions for use
Before receiving cabazitaxel, you will be given anti-allergic medicines to reduce the risk of allergic reactions.
- This medicine will be given to you by a doctor or nurse.
- This medicine must be prepared (diluted) before administration. This leaflet provides practical information for handling and administration of this medicine for doctors, nurses, and pharmacists.
- This medicine will be given to you in a hospital through a drip (infusion) into one of your veins (intravenously) over approximately 1 hour.
- As part of your treatment, you will also take a corticosteroid medicine (prednisone or prednisolone) by mouth every day.
How much and how often it is given
- The usual dose depends on your body surface area. Your doctor will calculate your body surface area in square meters (m2) and decide the dose you should receive.
- Usually, you will receive an infusion every 3 weeks.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Your doctor will discuss this with you and explain the risks and potential benefits of your treatment.
Seek immediate medical attention if you notice any of the following side effects:
- fever (high temperature). This is very common (may affect more than 1 in 10 people).
- severe loss of body fluids (dehydration). This is common (may affect up to 1 in 10 people). This can happen if you have severe or persistent diarrhea, or if you have been vomiting.
- severe stomach pain or stomach pain that does not go away. This can happen if you have a perforation in the stomach, esophagus, or intestine (gastrointestinal perforation). This can be life-threatening.
If you notice any of the above, tell your doctor immediately.
Other side effects include:
Very common(may affect more than 1 in 10 people):
- reduction in red blood cells (anemia), or white blood cells (which are important for fighting infections).
- reduction in platelets (which can result in an increased risk of bleeding).
- loss of appetite (anorexia).
- stomach upset, including nausea, vomiting, diarrhea, or constipation.
- back pain.
- blood in the urine.
- fatigue, weakness, or lack of energy.
Common(may affect up to 1 in 10 people):
- altered taste.
- shortness of breath.
- cough.
- abdominal pain.
- temporary hair loss (in most cases, hair grows back normally).
- joint pain.
- urinary tract infection.
- low white blood cell count associated with fever and infections.
- numbness, tingling, burning, or decreased sensation in hands and feet.
- dizziness.
- headache.
- increased or decreased blood pressure.
- indigestion, heartburn, or belching.
- stomach pain.
- hemorrhoids.
- muscle spasms.
- urinating frequently or with pain.
- urinary incontinence.
- kidney problems.
- mouth or lip ulcers.
- infections or risk of infections.
- high blood sugar levels.
- insomnia.
- mental confusion.
- feeling anxious.
- strange sensation or loss of sensation or pain in hands and feet.
- balance problems.
- rapid or irregular heartbeat.
- blood clots in the legs or lungs.
- flushing of the skin.
- mouth or throat pain.
- rectal bleeding.
- muscle disorders, weakness, or pain.
- swelling of feet or legs.
- chills.
- nail disorders (change in nail color; nails may come off)
Uncommon(may affect up to 1 in 100 people):
- low potassium levels in the blood.
- ringing in the ears.
- feeling of heat in the skin
- red skin.
- bladder inflammation, which can occur when your bladder has previously been exposed to radiation therapy (radiation recall cystitis).
Frequency not known(cannot be estimated from the available data):
- interstitial lung disease (inflammation of the lungs causing cough and difficulty breathing).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cabazitaxel Aurovit
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after EXP. The expiry date refers to the last day of the month shown.
This medicine does not require any special storage conditions.
In the section “Practical information for doctors or healthcare professionals on the preparation, administration, and handling of Cabazitaxel Aurovit 20 mg/ml concentrate for solution for infusion”, information is included on the storage and use of this medicine once it has been diluted and is ready for use.
Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition ofCabazitaxel Aurovit
- The active ingredient is cabazitaxel. One ml of concentrate contains 20 mg of cabazitaxel. Each 3 ml vial of concentrate contains 60 mg of cabazitaxel.
- The other components are: polysorbate 80, citric acid, and anhydrous ethanol (see Section 2. "Cabazitaxel Aurovit contains ethanol (alcohol)".
Appearance of the Product and Container Contents
Cabazitaxel Aurovit is a concentrate for solution for infusion (sterile concentrate). It is a clear, oily solution, yellow to yellowish-brown in color.
Each vial contains 3 ml of sterile concentrate.
The available pack size is 1 vial.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Eugia Pharma (Malta) Limited
Vault 14, Level 2, Valletta Waterfront
Floriana, FRN 1914
Malta
Manufacturer
AqVida GmbH
Kaiser-Wilhelm-Str. 89
20355 Hamburg
Germany
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
Aurovitas Spain, S.A.U.
Avda. de Burgos, 16-D
28036 Madrid
Spain
Date of Last Revision of this Leaflet:November 2023
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
This information is intended only for healthcare professionals:
"Practical Information for Doctors or Healthcare Professionals on the Preparation, Administration, and Handling of Cabazitaxel Aurovit 20 mg/ml Concentrate for Solution for Infusion"
This information complements sections 3 and 5 for the user.
It is essential to read the entire content of this procedure before preparing the infusion solution.
Incompatibilities
This medicinal product must not be mixed with other medicinal products except those used for dilution.
Shelf-Life and Special Precautions for Storage
Storage of the unopened vial
This medicinal product does not require special storage conditions.
Storage after opening the vial
The chemical and physical stability of the opened vial has been demonstrated for 4 weeks at 2°C-8°C.
From a microbiological point of view, the vial should be used immediately after opening. If not used immediately, the storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at 2°C-8°C, unless the dilution has been prepared in controlled and validated aseptic conditions.
Storage after dilution in 5% glucose solution or 0.9% sodium chloride solution
The chemical and physical stability of the infusion solution, in a non-PVC infusion bag/bottle, has been demonstrated for 48 hours at 25°C and for 14 days at 2°C-8°C.
From a microbiological point of view, the infusion solution should be used immediately. If not used immediately, the storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at 2°C-8°C, unless the dilution has been prepared in controlled and validated aseptic conditions.
Precautions for Preparation and Administration
As with other antineoplastic agents, it is recommended to handle the preparation and administration of cabazitaxel solutions with caution, taking into account the use of safety devices, personal protective equipment (e.g., gloves), and preparation procedures.
If, at any stage of preparation, cabazitaxel comes into contact with the skin, wash immediately and thoroughly with water and soap. If it comes into contact with mucous membranes, wash immediately and thoroughly with water.
Cabazitaxel Aurovit should only be prepared and administered by personnel trained in the handling of cytotoxic agents. Pregnant workers should not handle it.
Preparation Steps
DO NOT USE this medicinal product, which consists of a single vial with 3 ml of concentrate (60 mg/3 ml), with other medicinal products containing cabazitaxel in 2 vials (concentrate and solvent) or with other medicinal products containing cabazitaxel in a vial (only concentrate) but with a different concentration.
Each vial is for single use and should be used immediately. Any unused solution should be discarded.
The dilution process to prepare the infusion solution should be carried out aseptically.
Preparation of the Infusion Solution
Step 1:
It may be necessary to use more than 1 vial of cabazitaxel concentrate to obtain the required dose for the patient.
Aseptically withdraw the necessary volume from the Cabazitaxel Aurovit vial (which contains 20 mg/ml of cabazitaxel) using a graduated syringe with a fine needle.
For example, a dose of 45 mg of cabazitaxel would require 2.25 ml of Cabazitaxel Aurovit.
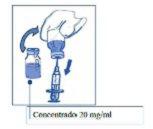
Step 2:
Inject into a sterile non-PVC container of 5% glucose solution or 0.9% sodium chloride solution for infusion. The concentration of the infusion solution should be between 0.10 mg/ml and 0.26 mg/ml.
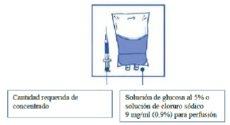
Step 3:
Remove the syringe and mix the contents of the infusion bag or bottle manually by rocking motion.

Step 4:
As with all parenteral products, the resulting infusion solution should be inspected visually before use. Since the infusion solution is supersaturated, it may crystallize over time. In this case, it should not be used and should be discarded.
Method of Administration
The infusion solution should be used immediately. However, the in-use storage time may be longer as explained in "Shelf-Life and Special Precautions for Storage".
This medicinal product is administered by infusion over 1 hour.
The use of an in-line filter with a 0.22 micrometer nominal pore size (also known as 0.2 micrometers) is recommended during administration.
PVC infusion containers or polyurethane infusion sets should not be used for the preparation and administration of cabazitaxel.
Cabazitaxel should not be mixed with other medicinal products except those mentioned.
Disposal
Disposal of unused medicinal product and all materials that have come into contact with it should be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CABAZITAXEL AUROVIT 20 mg/mL concentrate for infusion solutionDosage form: INJECTABLE PERFUSION, 20 mg/mlActive substance: cabazitaxelManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE PERFUSION, 60 mgActive substance: cabazitaxelManufacturer: Reddy Pharma Iberia S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 10 mg/mlActive substance: cabazitaxelManufacturer: Ever Valinject GmbhPrescription required
Online doctors for CABAZITAXEL AUROVIT 20 mg/mL concentrate for infusion solution
Discuss questions about CABAZITAXEL AUROVIT 20 mg/mL concentrate for infusion solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






