
BUDESONIDE ALDO UNION 200 micrograms/actuation PRESSURED INHALER SUSPENSION

How to use BUDESONIDE ALDO UNION 200 micrograms/actuation PRESSURED INHALER SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Budesonide Aldo-Union 200 micrograms and what is it used for
- What you need to know before taking Budesonide Aldo-Union 200 micrograms
- How to take Budesonide Aldo-Union 200 micrograms
- Possible side effects
- Storage of Budesonide Aldo-Union 200 micrograms
- Contents of the pack and additional information
Introduction
Leaflet: information for the user
Budesonide Aldo-Union 200 micrograms/puff
inhalation suspension in pressurized container
Read this leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet.
Contents of the leaflet
- What is Budesonide Aldo-Union 200 micrograms and what is it used for
- What you need to know before taking Budesonide Aldo-Union 200 micrograms
- How to take Budesonide Aldo-Union 200 micrograms
- Possible side effects
- Storage of Budesonide Aldo-Union 200 micrograms
- Contents of the pack and additional information
1. What is Budesonide Aldo-Union 200 micrograms and what is it used for
Budesonide is a non-halogenated corticosteroid effective in the treatment of asthma due to its anti-inflammatory capacity.
It is indicated in:
- Treatment of bronchial asthma, in patients who have not previously responded to therapy with bronchodilators and/or antiallergics.
2. What you need to know before taking Budesonide Aldo-Union 200 micrograms
Do not useBudesonide Aldo-Union 200 micrograms:
- If you are allergic to the active substance or to any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Budesonide Aldo-Union 200 micrograms.
Be particularly careful with this medicine:
This medicine is used as maintenance treatment and should not be used to treat acute asthma attacks (sudden attacks of shortness of breath and wheezing).
Do not stop treatment with this medicine suddenly.
You should be aware of the possibility of paradoxical bronchospasm with an increase in wheezing after administration. If wheezing appears suddenly after using this medicine, stop using it and consult your doctor immediately, as you may need to change treatment.
Contact your doctor if you experience blurred vision or other visual disturbances.
Important information about some of the components ofBudesonide Aldo-Union 200 micrograms:
Athletes are informed that this medicine contains a component that may result in a positive doping test.
Children and adolescents
There is another presentation, Budesonide 50 micrograms Aldo-Union, specifically indicated for children.
Other medicines andBudesonide Aldo-Union 200 micrograms
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medicine.
Some medicines may increase the effects of budesonide, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
This medicine will only be administered during pregnancy or breastfeeding when, in the doctor's opinion, the expected benefit to the mother is greater than any possible risk to the fetus.
Driving and using machines
No effects on the ability to drive or use machines have been described.
Budesonide Aldo-Union 200 micrograms contains ethanol:
This medicine contains 0.5 mg of alcohol (ethanol) per inhalation. The amount per inhalation of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect.
3. How to take Budesonide Aldo-Union 200 micrograms
Remember to use your medicine.
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
This medicine is administered by inhalation.
The dosage should be individualized.
Unless your doctor has given you different instructions, follow the recommended dosage:
Use in adults
Adults:200-1600 micrograms daily, divided into 2-4 administrations.
Use in children and adolescents
Children 2-7 years:200-400 micrograms daily, divided into 2-4 administrations.
Children from 7 years:200-800 micrograms daily, divided into 2-4 administrations.
Once the desired clinical effects are achieved, your doctor may gradually reduce the dose to the minimum amount necessary to control the symptoms.
If you think the effect of this medicine is too strong or too weak, let your doctor know. Do not change your dose without talking to your doctor first.
Rinse your mouth with water after using the inhaler, do not swallow. This will make it less likely to produce side effects in the mouth or throat.
Instructions for the correct administration of the preparation:
Before using the medicine, check the expiration date.
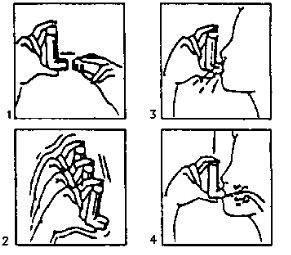
- Remove the cap (fig.1). If it is a new inhaler or has not been used for several days, shake the aerosol (fig. 2) and perform a puff to ensure the proper functioning of the inhaler. If the inhaler is used regularly, proceed to the next instructions:
- Shake the inhaler (fig. 2).
- Remove as much air as possible from your lungs.
- Attach the aerosol to your mouth according to the position indicated in the drawing (fig. 3).
- Take a deep breath.
You should press, according to the arrows in the drawing (fig. 4), the device while taking this breath.
- Remove the aerosol from your mouth and try to hold your breath for a few seconds.
- You should periodically clean the oral adapter of the aerosol. To do this, remove the adapter from the aerosol and wipe it with a cloth or paper towel.
- Store with the cap on and protect it from dust and dirt.
Your doctor should check that you know how to use the inhaler and synchronize your breath with the puff. Optionally, inhalation chambers can be used to achieve better use of the dose and facilitate the arrival of the medicine to the lungs.
The inhaler has a dose indicator that can be seen through a small hole or window on the actuator and indicates how many applications are left. In a new inhaler, you can read "120" or "200" (depending on the size of the container prescribed) through the window of the actuator. These numbers correspond to the doses left in the inhaler. As you use the inhaler, the dose indicator rotates downward every 5-7 puffs until it reaches 0.
When there are approximately 40 doses left, the indicator changes from green to red (see figure 5) to remind the patient to consult their doctor if they need to continue treatment or need a new prescription. Discard the inhaler once the indicator reaches "0".

(figure 5)
If you use moreBudesonide Aldo-Union 200 microgramsthan you should
It is important that you take your dose exactly as your doctor has indicated. Do not increase or decrease your dose without medical supervision.
Although no toxic symptoms are expected in case of overdose or accidental ingestion, if you have inhaled more budesonide than you should, consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult the Toxicological Information Service. Phone 91 562 04 20.
If you observe symptoms such as edema, swelling of the face or moon face, etc., you should inform your doctor so that the appropriate measures can be taken.
If you forget to use Budesonide Aldo-Union 200 micrograms
Do not use a double dose to make up for forgotten doses.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Occasional cases of mild throat irritation, cough, and hoarseness have been described. Similarly, cases of overinfection by Candida in the oral cavity, pharynx, and larynx have been reported. In most cases, they respond to topical antifungal therapy without the need to interrupt treatment with budesonide. In exceptional cases, cutaneous allergic reactions (urticaria, dermatitis) associated with the use of topical corticosteroids have been described.
You should be aware of the possibility of paradoxical bronchospasm with an increase in wheezing after administration. If wheezing appears suddenly after using this medicine, stop using it and consult your doctor immediately, as you may need to change treatment.
Frequency not known (cannot be estimated from available data):
Sleep disturbances, depression or feeling of concern, restlessness, nervousness, excitability or irritability (these effects are more likely to occur in children), and blurred vision (see also section 2: Warnings and Precautions).
If you experience side effects, consult your doctor or pharmacist or nurse, even if they are not listed in this leaflet.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Budesonide Aldo-Union 200 micrograms
Keep this medicine out of the sight and reach of children.
Store below 30°C. Protect from direct sunlight and do not freeze or cool.
The container contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not puncture the container even if it appears to be empty.
Do not use this medicine after the expiration date stated on the carton after EXP:. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and additional information
Composition ofBudesonide Aldo-Union 200 micrograms.
- The active substance is budesonide.
- The other components (excipients) are oleic acid, ethanol, and Norflurane.
Appearance of the product and contents of the pack
Budesonide Aldo-Union 200 micrograms is presented as an inhalation suspension in a pressurized container with a dose indicator in a 6 ml container that allows 120 applications and a 10 ml container that allows 200 applications.
Marketing authorization holder and manufacturer
Laboratorio Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat (Barcelona)
Spain
Date of the last revision of this leaflet:March 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
- Country of registration
- Average pharmacy price13.33 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUDESONIDE ALDO UNION 200 micrograms/actuation PRESSURED INHALER SUSPENSIONDosage form: PULMONARY INHALATION, 0.25 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.25 mg budesonide/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for BUDESONIDE ALDO UNION 200 micrograms/actuation PRESSURED INHALER SUSPENSION
Discuss questions about BUDESONIDE ALDO UNION 200 micrograms/actuation PRESSURED INHALER SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















