BRIUMVI 150 MG CONCENTRATE FOR INFUSION SOLUTION

How to use BRIUMVI 150 MG CONCENTRATE FOR INFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
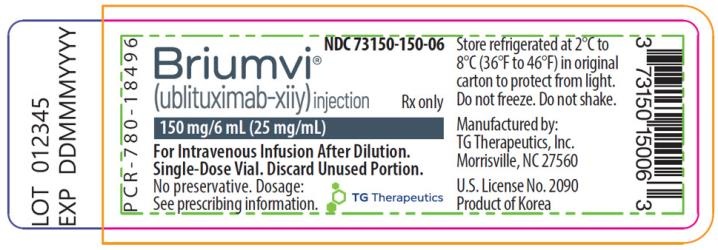
Briumvi 150 mg Concentrate for Solution for Infusion
Ublituximab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist, even if you think they are not serious. See section 4.
Contents of the pack
- What is Briumvi and what is it used for
- What you need to know before you are given Briumvi
- How Briumvi is given
- Possible side effects
- Storage of Briumvi
- Contents of the pack and other information
1. What is Briumvi and what is it used for
What is Briumvi
Briumvi contains the active substance ublituximab. It is a type of protein called a monoclonal antibody. Antibodies work by binding to specific targets in the body.
What is Briumvi used for
Briumvi is used to treat adults with relapsing forms of multiple sclerosis (MS), where the patient has relapses (exacerbations) followed by periods with milder or no symptoms.
What is multiple sclerosis
Multiple sclerosis (MS) affects the central nervous system, particularly the nerves in the brain and spinal cord. In MS, white blood cells called B lymphocytes, which are part of the immune system (the body's defense system), act incorrectly and attack a protective layer (called the myelin sheath) that surrounds nerve cells, causing inflammation and damage. The breakdown of the myelin sheath prevents the nerves from working properly and causes the symptoms of MS. The symptoms of MS depend on which part of the central nervous system is affected and can include problems with walking and balance, muscle weakness, numbness, double vision and blurred vision, coordination problems, and bladder problems.
In relapsing forms of MS, the patient has repeated episodes of symptoms (relapses) that can appear suddenly over a few hours or gradually over several days. The symptoms go away or improve between relapses, but damage can build up and lead to permanent disability.
How does Briumvi work
Briumvi works by binding to a target called CD20 on the surface of B lymphocytes. B lymphocytes are a type of white blood cell that is part of the immune system. In multiple sclerosis, the immune system attacks the protective layer that surrounds nerve cells. B lymphocytes are involved in this process. Briumvi acts on B lymphocytes and eliminates them, reducing the likelihood of relapse, alleviating symptoms, and slowing down the progression of the disease.
2. What you need to know before you are given Briumvi
You must not be given Briumvi
- if you are allergicto ublituximab or any of the other ingredients of this medicine (listed in section 6);
- if you have a severe infection;
- if you have been told you have serious problems with your immune system; or
- if you have cancer.
If you are not sure, consult your doctor before you are given Briumvi.
Warnings and precautions
Tell your doctor before you are given Briumviif you meet any of the following criteria. Your doctor may decide to postpone your treatment with Briumvi or that you cannot be given Briumvi if:
- you have an infection. Your doctor will wait until the infection is resolved before giving you Briumvi.
- you have ever had hepatitis Bor are a carrier of the hepatitis B virus. This is because medicines like Briumvi can cause the hepatitis B virus to become active again. Before treatment with Briumvi, your doctor will check if you are at risk of hepatitis B virus infection. Patients who have had hepatitis B or are carriers of the hepatitis B virus will have a blood test and will be monitored by a doctor for signs of hepatitis B virus infection.
- you have recently received or may receive a vaccine in the near future.
- you have canceror have had cancer in the past. Your doctor may decide to postpone treatment.
Infusion-related reactions
- The most common side effect of treatment with Briumvi is infusion-related reactions, a type of allergic reaction that occurs during or shortly after administration of a medicine. They can be serious.
- Symptoms of an infusion-related reaction can include, but are not limited to, the following:
- itching of the skin
- hives
- redness of the face or skin
- throat irritation
- breathing problems
- swelling of the tongue or throat
- wheezing
- chills
- fever
- headache
- dizziness
- feeling of fainting
- nausea
- abdominal pain (in the abdomen)
- rapid heartbeat
- Tell your doctor or nurse immediately if you have or think you may have an infusion-related reaction. Infusion-related reactions can occur during or up to 24 hours after the infusion.
- To reduce the risk of an infusion-related reaction, your doctor will give you other medicines before each Briumvi infusion (see section 3) and you will be closely monitored during the infusion.
- If you have an infusion-related reaction, your doctor may need to slow down or temporarily stop the infusion.
Infections
- Tell your doctor before you are given Briumvi if you have or think you may have an infection. Your doctor will wait until the infection is resolved before giving you Briumvi.
- You may be more likely to get infections with Briumvi. This is because the immune cells that Briumvi acts on also help to fight infections.
- Tell your doctor or nurse immediately if you have an infection or any of the following signs of infection during or after treatment with Briumvi:
- fever or chills
- persistent cough
- herpes (e.g. cold sores, shingles, or genital ulcers)
- Tell your doctor or nurse immediately if you think your MS is getting worse or if you notice any new symptoms. This is due to a rare and potentially life-threatening brain infection called progressive multifocal leukoencephalopathy (PML), which can cause symptoms similar to those of MS. PML can occur in patients treated with medicines like Briumvi and other medicines for the treatment of MS.
- Tell your partner or caregiverabout your treatment with Briumvi. They may notice symptoms of PML that you do not notice, such as memory loss, problems with thinking, difficulty walking, loss of vision, or changes in speech, which your doctor may need to investigate.
Vaccines
- Tell your doctor if you have recently received or may receive a vaccine in the near future.
- Your doctor will check if you need any vaccines before starting treatment with Briumvi. You should receive a type of vaccine called live or attenuated vaccines at least 4 weeks before starting treatment with Briumvi. During treatment with Briumvi, you should not receive live or attenuated vaccines until your doctor tells you that your immune system is no longer weak.
- Whenever possible, you should receive other types of vaccines called inactivated vaccines at least 2 weeks before starting treatment with Briumvi. If you wish to receive inactivated vaccines during treatment with Briumvi, consult your doctor.
Children and adolescents
The use of Briumvi is not indicated in children and adolescents under 18 years of age. This is because it has not been studied in this age group.
Other medicines and Briumvi
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. In particular, tell your doctor:
- if you are taking, have recently taken, or might take medicines that affect the immune system, such as chemotherapy, immunosuppressants (except corticosteroids), or other medicines used to treat MS. This is because they may have an additive effect on the immune system.
- if you are scheduled to receive a vaccine (see "Warnings and precautions" above).
If you meet any of these criteria (or are not sure), consult your doctor before you are given Briumvi.
Pregnancy and breastfeeding
- Tell your doctor before you are given Briumvi if you are pregnant, think you may be pregnant, or plan to become pregnant. This is because Briumvi may pass through the placenta and affect the fetus.
- Do not use Briumvi if you are pregnant unless you have discussed it with your doctor. Your doctor will weigh the benefit to you of taking Briumvi against the risk to the fetus.
- If you have a child and have received Briumvi during pregnancy, it is important that you tell your child's doctor that you have received Briumvi, so that the doctor can recommend when your child should be vaccinated.
- It is not known if Briumvi passes into breast milk. Consult your doctor about the best way to feed your child if you are given Briumvi.
Female contraception
If you are able to become pregnant (conceive), you must use contraceptive methods:
- during treatment with Briumvi and
- for at least 4 months after the last infusion of Briumvi.
Driving and using machines
Briumvi is unlikely to affect your ability to drive or use machines.
Briumvi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How Briumvi is given
Briumvi will be given to you by a doctor or a nurse with experience in the use of this treatment. You will be closely monitored while you are being given this medicine. This is in case you have any side effects.
You will always receive Briumvi through a drip (intravenous infusion).
Medicines that will be given to you before you receive Briumvi
Before you receive Briumvi, you will be given other medicines to prevent or reduce possible side effects such as infusion-related reactions (see sections 2 and 4 for information on infusion-related reactions).
You will receive a corticosteroid and an antihistamine before each infusion and you may also receive other medicines to reduce fever.
How much Briumvi you will receive and how often you will receive it
- The first dose of Briumvi will be 150 mg. This infusion will last 4 hours.
- The second dose of Briumvi will be 450 mg given 2 weeks after the first dose. This infusion will last 1 hour.
- Later doses of Briumvi will be 450 mg given 24 weeks after the first dose and every 24 weeks thereafter. These infusions will last 1 hour.
How Briumvi is given
- Briumvi will be given to you by a doctor or a nurse. Briumvi must be diluted before it is given. A healthcare professional will do the dilution. It will be given as an infusion into a vein (intravenous infusion).
- You will be closely monitored during the infusion of Briumvi and for at least 1 hour after the infusion of the first two infusions. This is in case you have any side effects such as an infusion-related reaction. The infusion can be slowed down, temporarily stopped, or permanently stopped if you have an infusion-related reaction, depending on its severity (see sections 2 and 4 for information on infusion-related reactions).
If you miss an infusion of Briumvi
- If you miss an infusion of Briumvi, consult your doctor to schedule it as soon as possible. Do not wait for the next scheduled infusion.
- To get the full benefit of Briumvi, it is important that you receive each infusion on time.
If you stop treatment with Briumvi
- It is important that you continue treatment while you and your doctor decide that it is being beneficial for you.
- Some side effects may be related to having low levels of B lymphocytes. After stopping treatment with Briumvi, you may experience these side effects until normal B lymphocyte levels are restored.
- Before you start taking other medicines, tell your doctor when you received your last infusion of Briumvi.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported with Briumvi:
Serious side effects
Infusion-related reactions
- Infusion-related reactions are the most common side effect of treatment with Briumvi (very common: may affect more than 1 in 10 people). In most cases, they are mild, but some can be serious.
- Tell your doctor or nurse immediately if you experience signs or symptoms of an infusion-related reaction during or up to 24 hours after the infusion. Symptoms can include, but are not limited to, the following:
- itching of the skin
- hives
- redness of the face or skin
- throat irritation
- breathing problems
- swelling of the tongue or throat
- wheezing
- chills
- fever
- headache
- dizziness
- feeling of fainting
- nausea
- abdominal pain (in the abdomen)
- rapid heartbeat
- If you have an infusion-related reaction, you will be given medicines to treat it and the infusion may need to be slowed down or stopped. When the reaction has stopped, the infusion can be restarted. If the infusion-related reaction is life-threatening, your doctor will permanently stop treatment with Briumvi.
Infections
- You may be more likely to get infections with Briumvi. Some of these can be serious. The following infections have been seen in patients with MS treated with Briumvi:
- Very common(may affect more than 1 in 10 people)
- upper respiratory tract infections (infections of the nose and throat)
- respiratory tract infections
- Common(may affect up to 1 in 10 people)
- lower respiratory tract infections (infections of the lungs such as bronchitis or pneumonia)
- herpes infections (cold sores, shingles, or genital ulcers)
- Tell your doctor or nurse immediately if you notice any of the following signs of infection:
- fever or chills
- persistent cough
- herpes (e.g. cold sores, shingles, or genital ulcers)
Your doctor will wait until the infection is resolved before giving you Briumvi.
Other side effects
Common(may affect up to 1 in 10 people)
- neutropenia (low levels of neutrophils, a type of white blood cell)
- pain in an extremity (arms or legs)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if you think they are not serious. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Briumvi
Keep in the refrigerator (between 2°C and 8°C).
Healthcare professionals will store Briumvi in the hospital or clinic under the following conditions:
- This medicine should not be used after the expiry date which is stated on the carton and on the label of the vial after "EXP". The expiry date is the last day of the month shown.
- This medicine should be stored in the refrigerator (between 2°C and 8°C). Do not freeze. The vial should be kept in the outer packaging to protect it from light.
It is recommended to use the product immediately after dilution. If not used immediately, the in-use storage times and conditions are the responsibility of the healthcare professional and normally should not exceed 24 hours between 2°C and 8°C and subsequently 8 hours at room temperature.
Medicines should not be disposed of via wastewater or household waste. This will help to protect the environment.
6. Container Contents and Additional Information
Briumvi Composition
- The active ingredient is ublituximab. Each vial contains 150 mg of ublituximab in 6 ml at a concentration of 25 mg/ml.
- The other components are sodium chloride, disodium citrate dihydrate, polysorbate 80, hydrochloric acid, and water for injectable preparations.
Product Appearance and Container Contents
- Briumvi is a solution that is between transparent and opalescent and between colorless and yellowish.
- It is presented as a concentrate for solution for infusion.
- This medicinal product is available in containers containing 1 vial (glass vial with 6 ml of concentrate).
Marketing Authorization Holder
Neuraxpharm Pharmaceuticals, S.L.
Avda. Barcelona 69
08970 Sant Joan Despí - Barcelona
Spain
Manufacturer
Millmount Healthcare
Block 7, City North Business Campus
Stamullen
Co. Meath
Ireland
K32 YD60
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Neuraxpharm Belgium Tel: +32 (0)2 732 56 95 | Lithuania Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Luxembourg Neuraxpharm France Tel: +32 474 62 24 24 |
Czech Republic Neuraxpharm Bohemia s.r.o. Tel: +420 739 232 258 | Hungary Neuraxpharm Hungary Kft. Tel: +36 30 464 6834 |
Denmark Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Sweden) | Malta Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Germany Neuraxpharm Arzneimittel GmbH Tel: +49 2173 1060 0 | Netherlands Neuraxpharm Netherlands B.V. Tel: +31 70 208 5211 |
Estonia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Norway Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Sweden) |
Greece Brain Therapeutics PC Tel: +30 210 993 1458 | Austria Neuraxpharm Austria GmbH Tel: +43 (0) 2236 320038 |
Spain Neuraxpharm Spain, S.L.U. Tel: +34 93 475 96 00 | Poland Neuraxpharm Polska Sp. z.o.o. Tel: +48 783 423 453 |
France Neuraxpharm France Tel: +33 1.53.62.42.90 | Portugal Neuraxpharm Portugal, Unipessoal Lda Tel: +351 910 259 536 |
Croatia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | Romania Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Ireland Neuraxpharm Ireland Ltd Tel: +353 (0)1 428 7777 | Slovenia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 |
Iceland Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Sweden) | Slovakia Neuraxpharm Slovakia a.s. Tel: +421 255 425 562 |
Italy Neuraxpharm Italy S.p.A. Tel: +39 0736 980619 | Finland Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 (Sweden) |
Cyprus Brain Therapeutics PC Tel: +30 210 993 1458 | Sweden Neuraxpharm Sweden AB Tel: +46 (0)8 30 91 41 |
Latvia Neuraxpharm Pharmaceuticals, S.L. Tel: +34 93 475 96 00 | United Kingdom (Northern Ireland) Neuraxpharm Ireland Ltd Tel: +353 (0)1 428 7777 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended for healthcare professionals only:
Read the summary of product characteristics or package leaflet for further information.
Dosage
- First and second doses
The first dose is administered as an intravenous infusion of 150 mg (first infusion), followed by an intravenous infusion of 450 mg, 2 weeks later (second infusion).
- Subsequent doses
Subsequent doses of Briumvi are administered as a single intravenous infusion of 450 mg every 24 weeks (Table 1). The first subsequent dose of 450 mg should be administered 24 weeks after the first infusion. A minimum interval of 5 months should be maintained between each dose of Briumvi.
Figure 1: Dosing and Schedule of Briumvi
First Infusion | Second Infusion | Subsequent Infusions |
Day 1
| Day 15
| Every 6 months
|
Treatment of IRs before Infusion
- Treatment should be initiated and supervised by a healthcare professional with experience and access to adequate medical support to treat serious reactions such as severe infusion-related reactions (IRs).
- Pre-medication for IRs
The following two medications should be administered as pre-medication before each Briumvi infusion to reduce the frequency and severity of IRs:
- 100 mg of methylprednisolone or 10-20 mg of dexamethasone (or equivalent) approximately 30-60 minutes before each Briumvi infusion;
- diphenhydramine approximately 30-60 minutes before each Briumvi infusion.
In addition, pre-medication with an antipyretic (e.g., paracetamol) may also be considered.
Dilution Instructions
- Briumvi should be prepared by a healthcare professional using aseptic technique. Do not shake the vial.
- The product is intended for single use.
- Do not use the solution if it shows a change in color or contains foreign particles.
- Briumvi should be diluted before administration. Briumvi solutions for intravenous administration are prepared by diluting the product in an infusion bag containing 0.9% isotonic sodium chloride. For the first infusion, dilute one vial of the product in the infusion bag (150 mg/250 ml) to a final concentration of approximately 0.6 mg/ml. For subsequent infusions, dilute three vials of the product in the infusion bag (450 mg/250 ml) to a final concentration of approximately 1.8 mg/ml.
- Before starting the intravenous infusion, the contents of the infusion bag should be at room temperature.
Method of Administration
- After dilution, Briumvi is administered as an intravenous infusion through a dedicated line.
- Briumvi infusions should not be administered as a slow intravenous infusion or bolus.
Table 1: Dosing and Schedule of Briumvi
Quantity and Volume | Infusion Rate | Duration 1 | |
First Infusion | 150 mg in 250 ml |
2 hours. | 4 hours |
Second Infusion (2 weeks later) | 450 mg in 250 ml |
30 minutes. | 1 hour |
Subsequent Infusions (every 24 weeks) | 450 mg in 250 ml |
30 minutes. | 1 hour |
1The infusion duration may be longer if the infusion is interrupted or slowed down.
2The first subsequent dose should be administered 24 weeks after the first infusion.
Treatment of IRs during and after Infusion
Patient should be monitored during the infusion and for at least one hour after the end of the first two infusions.
During Infusion
- Infusion adjustments in case of IRs
In case of IRs during an infusion, the following adjustments should be considered.
Potentially life-threatening IRs
If there are signs of a potentially life-threatening or debilitating IR during an infusion, the infusion should be stopped immediately and the patient should receive appropriate treatment. In these patients, treatment with Briumvi should be permanently discontinued (see section 4.3).
Severe IRs
If a patient experiences a severe IR, the infusion should be stopped immediately and the patient should receive symptomatic treatment. The infusion should only be restarted once all symptoms have resolved. When restarting the infusion, start at half the infusion rate applied at the time of the IR. If the infusion rate is tolerated, increase the rate as described in Table 1.
Mild to moderate IRs
If a patient experiences a mild to moderate IR, the infusion rate should be reduced to half the infusion rate applied at the time of the IR. This reduced infusion rate should be maintained for at least 30 minutes. If the reduced infusion rate is tolerated, the infusion rate may be increased as described in Table 1.
After Infusion
- Patient should be observed for at least one hour after the end of the first two infusions for signs of IRs.
- Physicians should inform patients that an IR may occur within 24 hours after the infusion.
Shelf Life
Unopened vial
3 years
Diluted solution for intravenous infusion
- Chemical and physical stability has been demonstrated for 24 hours at 2°C to 8°C and subsequently for 8 hours at room temperature.
- From a microbiological point of view, the prepared infusion solution should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and normally should not exceed 24 hours at 2°C to 8°C and subsequently 8 hours at room temperature, unless the dilution has been made in controlled and validated aseptic conditions.
- If an intravenous infusion cannot be completed on the same day, the remaining solution should be discarded.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRIUMVI 150 MG CONCENTRATE FOR INFUSION SOLUTIONDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for BRIUMVI 150 MG CONCENTRATE FOR INFUSION SOLUTION
Discuss questions about BRIUMVI 150 MG CONCENTRATE FOR INFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions