
BREAKYL 1200 micrograms ORAL FILM

How to use BREAKYL 1200 micrograms ORAL FILM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Breakyl 200 micrograms buccal film
Breakyl 400 micrograms buccal film
Breakyl 600 micrograms buccal film
Breakyl 800 micrograms buccal film
Breakyl 1200 micrograms buccal film
fentanyl
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Breakyl and what is it used for
- What you need to know before you use Breakyl
- How to use Breakyl
- Possible side effects
- Storing Breakyl
- Contents of the pack and further information
1. What is Breakyl and what is it used for
Breakyl buccal film contains the active substance fentanyl, which is a potent medicine for the treatment of pain, known as an opioid. Breakyl is indicated for the treatment of breakthrough cancer pain in adult patients. Breakthrough pain is a sudden increase in pain. This can occur even if you have been taking regular pain-relieving medicines, such as opioids, for your ongoing cancer pain.
Breakyl should only be used if you are already taking and are tolerant to regular opioid therapy for your cancer pain, such as morphine, oxycodone, or transdermal fentanyl, for at least one week.
2. What you need to know before you use Breakyl
Do not use Breakyl
- If you are allergic (hypersensitive) to fentanyl or any of the other ingredients of this medicine (listed in section 6).
- If you are currently taking monoamine oxidase inhibitors (MAOIs) (used for severe depression) or have taken them in the last 2 weeks.
- If you are taking a medicine that contains sodium oxybate.
- If you have severe breathing problems or severe obstructive lung disease (such as severe asthma).
- If you are not regularly using a prescribed opioid medicine (such as codeine, fentanyl, hydromorphone, morphine, oxycodone, pethidine) every day at the same time for at least one week to control your persistent pain. If you have not been using these medicines, do not useBreakyl as its use may increase the risk that your breathing becomes slower and/or shallower, and even stops.
- If you have short-term pain that is not breakthrough pain.
Warnings and precautions
Keep this medicine out of the sight and reach of other people, especially children, to avoid accidental intake (for more information, see section 5. Storing Breakyl).
Talk to your doctor before you start using Breakyl if you have any of the following problems, as your doctor will need to consider them when prescribing your dose:
- Your other opioid pain medicine for your ongoing cancer pain has not yet been stabilized.
- You have any breathing problems.
- You have had a head injury or your doctor has told you that you have increased pressure in your head.
- You have heart problems, especially slow heart rate or other heart problems.
- You have low blood pressure, especially if it is caused by a reduced amount of fluid in the circulation.
- You have liver or kidney problems, as these organs have an effect on how your body gets rid of the medicine.
- You have mouth ulcers.
- You have had addiction or been dependent on opioids, alcohol, prescription medicines, or illegal drugs.
- You are taking antidepressants or antipsychotics, see section “Other medicines and Breakyl”.
See additional information in section 3.
Sleep apnea syndrome
Breakyl may cause sleep apnea syndrome, including sleep apnea (pauses in breathing while sleeping) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing while sleeping, waking up during the night due to lack of air, difficulty staying asleep, or excessive sleepiness during the day. If you or another person notice these symptoms, contact your doctor to assess the possibility of reducing the dose.
Talk to your doctor if, while using Breakyl, you:
- Feel pain or increased sensitivity to pain (hyperalgesia) that does not respond to a higher dose of the medicine as prescribed by your doctor.
- Experience a combination of the following symptoms: nausea, vomiting, loss of appetite, fatigue, weakness, dizziness, and low blood pressure. Together, these symptoms can be a sign of a potentially life-threatening condition called adrenal insufficiency, in which the adrenal glands do not produce enough hormones.
- Have ever had adrenal insufficiency or a lack of sex hormones (androgen deficiency) with the use of opioids.
Long-term use and tolerance
This medicine contains fentanyl, an opioid. Repeated use of opioid pain-relievers can make the medicine less effective (your body gets used to it, which is known as pharmacological tolerance). You may also become more sensitive to pain when using Breakyl. This is known as hyperalgesia. Increasing the dose of Breakyl may continue to reduce the pain for a while, but it can also be harmful. If you notice that the medicine is becoming less effective, talk to your doctor. Your doctor will decide whether it is best for you to increase the dose or gradually reduce the use of Breakyl.
Dependence and addiction
This medicine contains fentanyl, which is an opioid. It can cause dependence and/or addiction. |
Repeated use of Breakyl can also lead to dependence, abuse, and addiction, which could result in a potentially life-threatening overdose. The risk of these side effects may be greater with higher doses and longer use. Dependence or addiction can cause a feeling of loss of control over the amount of medicine you need to use or how often you need to use it. You may feel the need to keep using the medicine even if it does not help relieve the pain.
The risk of dependence or addiction varies from person to person. The risk of becoming dependent on or addicted to Breakyl may be greater if:
- you or a family member have abused alcohol or have been dependent on it, prescription medicines, or illegal drugs (“addiction”).
- you smoke.
- you have ever had mood problems (depression, anxiety, or personality disorder) or have been treated by a psychiatrist for other mental health problems.
If you notice any of the following symptoms while using Breakyl, it could be a sign of dependence or addiction.
- you need to use the medicine for longer than prescribed by your doctor.
- you need to use a higher dose than recommended.
- you are using the medicine for reasons other than those prescribed, for example, “to feel calm” or “to help you sleep”.
- you have repeatedly tried to stop using the medicine or control its use but have been unsuccessful.
- you feel unwell when you stop using the medicine (for example, nausea, vomiting, diarrhea, anxiety, shivering, shaking, and sweating), and you feel better once you take it again (“withdrawal symptoms”).
If you notice any of these signs, talk to your doctor to determine the best course of treatment for you, when it is appropriate to stop the medicine, and how to do it safely.
Children and adolescents
Do not give this medicine to children aged 0 to 18 years.
Breakyl contains fentanyl in an amount that can be fatal for a child. Therefore, Breakyl must be kept out of the sight and reach of children at all times.
If you are an athlete, you should be aware that this product may produce a positive reaction in anti-doping tests.
Other medicines and Breakyl
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Do not use Breakyl if you are currently taking, or have taken in the last 2 weeks, monoamine oxidase inhibitors (MAOIs) (used for severe depression).
If you are taking any of the following medicines, talk to your doctor or pharmacist before you start using Breakyl: Any medicine that makes you feel drowsy or sleepy, such as:
- sleeping pills,
- medicines for treating anxiety, nervousness, depression,
- medicines for treating tension or stiffness in the muscles (muscle relaxants),
- medicines for treating allergies (antihistamines).
Medicines that can interfere with how (the enzyme CYP3A4 in) your body breaks down Breakyl, as they may increase the levels of fentanyl in your blood. This can lead to increased or prolonged effects of Breakyl and may cause potentially life-threatening breathing problems. These medicines include, for example:
- Medicines for treating bacterial infections (such as erythromycin, clarithromycin, telithromycin).
- Medicines for treating fungal infections (such as ketoconazole, itraconazole, fluconazole).
- Medicines for treating viral infections, including HIV (such as ritonavir, indinavir, nelfinavir, saquinavir).
- Medicines for treating cardiovascular diseases (such as diltiazem, verapamil).
- Medicines for treating severe nausea (such as aprepitant, dronabinol).
- Medicines for treating depression (such as fluoxetine).
- Medicines that inhibit the production of stomach acid (such as cimetidine).
The risk of side effects increases if you are taking certain antidepressants or antipsychotics. Breakyl may interact with these medicines and may cause changes in mental status (such as agitation, hallucinations, coma) and other effects such as body temperature above 38°C, increased heart rate, unstable blood pressure, and exaggerated reflexes, muscle stiffness, lack of coordination, and/or gastrointestinal symptoms (such as nausea, vomiting, diarrhea). Your doctor will tell you if Breakyl is suitable for you.
Medicines that can increase how (the enzyme CYP3A4 in) your body breaks down fentanyl, reducing the effectiveness of Breakyl, such as:
- sleeping pills or sedatives (such as phenobarbital),
- medicines for controlling seizures/epileptic fits (such as carbamazepine, phenytoin, oxcarbazepine),
- medicines for controlling the spread of viruses (such as efavirenz, nevirapine),
- anti-inflammatory or immunosuppressive medicines (such as glucocorticoids),
- medicines for treating diabetes (such as pioglitazone),
- antibiotics for treating tuberculosis (such as rifabutin, rifampicin),
- psychostimulant medicines (such as modafinil),
- medicines for treating depression (such as St. John’s Wort).
If you stop treatment or reduce the dose of these active substances during therapy with Breakyl, inform your doctor. Your doctor will closely monitor signs of opioid toxicity and may adjust the dose of Breakyl accordingly.
Using Breakyl and sedative medicines such as benzodiazepines or related medicines at the same time increases the risk of drowsiness, difficulty breathing (respiratory depression), coma, and can put your life at risk. Therefore, their combined use should be avoided if other treatment options are possible.
However, if Breakyl is prescribed with sedative medicines, your doctor will limit the dose and duration of treatment.
Tell your doctor about all sedative medicines you are taking and closely follow your doctor’s dose recommendation. It may be useful to inform friends or family members who are aware of the signs and symptoms mentioned above. Contact your doctor when you experience these symptoms.
If you take certain types of powerful painkillers, known as partial agonist/antagonists, such as buprenorphine, nalbuphine, and pentazocine (medicines for treating pain), you may experience withdrawal symptoms (nausea, vomiting, diarrhea, anxiety, shivering, shaking, and sweating) while using these medicines.
Using Breakyl with food, drinks, and alcohol
Avoid drinking alcohol, as alcohol may have an additional sedative and depressant effect on your breathing. Do not drink grapefruit juice, as it may slow down how your body breaks down fentanyl, which can lead to increased or prolonged effects of Breakyl, causing potentially life-threatening breathing problems.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You should not use Breakyl if you are pregnant, unless you have discussed it with your doctor. You should not use Breakyl during childbirth, as fentanyl may cause breathing difficulties in the newborn.
Fentanyl can pass into breast milk and cause side effects in the baby. Do not use Breakyl if you are breastfeeding. You should not start breastfeeding until at least 5 days after the last dose of Breakyl.
Driving and using machines
Ask your doctor if it is safe for you to drive a vehicle or use machines in the hours following the use of Breakyl.
Opioid pain-relievers, such as fentanyl, may affect your mental and/or physical ability to perform tasks that require attention, such as driving or operating machinery. You should not drive or use machines if you feel drowsy, dizzy, or experience blurred or double vision, or have difficulty concentrating while using Breakyl.
Breakyl contains propylene glycol (E1520), sodium benzoate (E211), methyl parahydroxybenzoate (E218), and propyl parahydroxybenzoate (E216).
Sodium benzoate may be slightly irritating to the skin, eyes, and mucous membranes. Methyl parahydroxybenzoate and propyl parahydroxybenzoate may cause allergic reactions (which may be delayed). Propylene glycol may cause skin irritation. This medicine contains less than 1 mmol of sodium (23 mg) per buccal film, which is essentially sodium-free.
3. How to use Breakyl
Before starting treatment and on a regular basis during treatment, your doctor will also explain what you can expect from using Breakyl, when and for how long you should use it, when you should contact your doctor, and when you should stop using it (see also section 2).
Follow your doctor's instructions for administering Breakyl exactly. Consult your doctor or pharmacist if you have any doubts.
Dosage
When you start using Breakyl, your doctor will determine the dose that will relieve your breakthrough pain (dose titration) together with you. This step is necessary because it is not possible to predict the dose of Breakyl that will be effective in your individual case based on your daily dose of opioids for the treatment of chronic cancer pain or any other medication you may have taken previously for breakthrough cancer pain.
For dose titration, the dose is gradually increased. Once the dose that provides you with adequate pain relief within 30 minutes is reached and any potential side effects are acceptable to you, you will have identified the effective dose. It is essential that you strictly follow your doctor's instructions.
Usually, the following procedure will be used for Dose Titration.
Dose Titration
You should start with an initial dose of 200 micrograms of Breakyl.
Contact your doctor if you do not achieve adequate relief from your breakthrough pain 30 minutes after applying the dose of Breakyl. If you have tolerated the dose, your doctor will recommend treating the next episode of breakthrough pain with the next higher dose of Breakyl. Your doctor may gradually increase the dose from 200 to 400 and 600 micrograms up to 800 micrograms.
By applying a combination of Breakyl buccal films simultaneously, it is possible to achieve the following higher doses:
1 Breakyl 200 buccal film is equivalent to a dose of 200 micrograms
2 Breakyl 200 buccal films are equivalent to a dose of 400 micrograms
3 Breakyl 200 buccal films are equivalent to a dose of 600 micrograms
4 Breakyl 200 buccal films are equivalent to a dose of 800 micrograms
If the combination of 4 buccal films at the same time (800 micrograms) is not sufficient to relieve the pain, your doctor may prescribe Breakyl 1200 micrograms. This is the maximum available dose of Breakyl.
Once you have identified the effective dose, your doctor will provide you with a prescription for this dose for the treatment of subsequent episodes of breakthrough pain, using this established dose.
Breakyl should be used only once per episode of breakthrough pain and you should wait at least 4 hours before using the next dose of Breakyl.
If you do not achieve adequate pain relief within 30 minutes of applying a dose of Breakyl, you may use another rescue medication for breakthrough pain if your doctor has instructed you to do so.
Administration Frequency
You should not take more than four doses of Breakyl per day.
Dose Adjustment
Inform your doctor immediately if you experience more than four episodes of breakthrough pain per day. In this case, your doctor may choose to increase your medication for persistent cancer pain. When your persistent cancer pain has been controlled, your doctor may need to adjust the dose of Breakyl again. To achieve the best results, inform your doctor about the evolution of your pain and the action of Breakyl, so that the dose can be adjusted if necessary.
Do not modify the doses of Breakyl or your regular opioid therapy on your own. Dose changes must be indicated and controlled by your doctor.
Administration Methods
The Breakyl buccal film should be applied to the buccal mucosa. When you attach the buccal film to the inside of your cheek, fentanyl is absorbed directly through the lining of the mouth into the bloodstream.
- Open the Breakyl package immediately before using it, as indicated on the package instructions;
- Use your tongue to moisten the inside of your cheek, or rinse your mouth with water to moisten the area where Breakyl will be placed;
- With dry hands, place the Breakyl buccal film between your index finger and thumb, with the pink side facing your thumb (Figure 1);
- Carefully place the pink side of the Breakyl buccal film against the inside of your cheek (Figure 2);
- Press and hold it in place for at least 5 seconds, until it adheres firmly. At this point, the white side of the buccal film should be visible (Figure 3);
- If you apply more than one Breakyl buccal film at the same time, make sure each film adheres directly to the buccal mucosa. To avoid overlapping, you can apply the films to both sides - left and right - of the buccal mucosa.
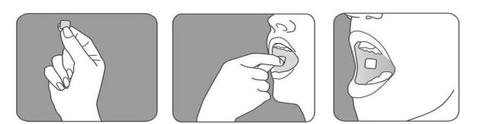
Figure 1 Figure 2 Figure 3
The Breakyl buccal film should remain in place on its own after this period.
You can drink liquids after 5 minutes.
Usually, the buccal film will dissolve completely within 15 to 30 minutes after application. In isolated cases, complete dissolution may take more than 30 minutes, but this does not affect the absorption of fentanyl.
Avoid handling the buccal film with your tongue or fingers. You should not eat while the film has not dissolved completely.
Do not chew or swallow the Breakyl film. If you do, you may get less relief from your breakthrough pain.
If you use more Breakyl than you should or if you think someone has used Breakyl accidentally
If after using Breakyl you start to feel very sleepy, remove the Breakyl buccal film from your mouth as soon as possible or even parts of it, and seek help from another person.
If you have taken more Breakyl than you should, you or your caregiver should contact your doctor, hospital, or emergency service, so they can assess the risk and indicate what to do.
Overdose symptoms may be:
- excessive drowsiness,
- dizziness,
- nausea or vomiting,
- very slow and/or shallow breathing,
- or decreased body temperature, slow heart rate, difficulty coordinating arms and legs.
An overdose can also cause a brain disorder known as toxic leukoencephalopathy.
If someone has accidentally used Breakyl, they may experience the same symptoms as those described for overdose.
At the beginning of treatment, these symptoms may appear if your dose of Breakyl is too high or if you use too much Breakyl. You and your caregiver should discuss with your doctor the immediate actions to be taken in this case.
Note for caregivers:
If you notice that the patient taking Breakyl or someone who accidentally took Breakyl, even if it was not prescribed to them, has slow and/or shallow breathing, or if it is very difficult to wake them up, take the following actions immediately:
- If the Breakyl buccal film is still attached to the inside of the patient's cheek, remove it from the patient's mouth as soon as possible, or even parts of it.
- Call emergency services.
- While waiting for emergency help:
- If the person is asleep, wake them up by calling their name and moving their arms or shoulders.
- If the person appears to be breathing slowly, force them to breathe every 5-10 seconds.
- If the person has stopped breathing, apply mouth-to-mouth breathing until help arrives.
If you stop treatment with Breakyl
You should stop using Breakyl when you no longer have breakthrough pain. However, you should continue taking your usual opioid medications to treat persistent cancer pain as recommended by your doctor. You may experience withdrawal symptoms similar to the possible side effects of Breakyl when you stop treatment with Breakyl. If you have withdrawal symptoms or are concerned about pain relief, consult your doctor, who will assess whether you need any medication to reduce or suppress withdrawal symptoms.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Breakyl can cause side effects, although not everyone gets them.
The most serious side effects are shallow breathing, low blood pressure, and shock. If you feel very sleepy or your breathing is slow and/or shallow, you or your caregiver should contact your doctor and call emergency services immediately. If the buccal film is still attached to your cheek, remove it as soon as possible, or even parts of it.
The most common adverse reactions observed have been nausea, drowsiness, and dizziness.
Since patients using Breakyl also take regular opioid therapies such as morphine, oxycodone, or transdermal fentanyl for their persistent pain, side effects caused by any of the treatments may occur. Therefore, it is not possible to clearly distinguish the effects of Breakyl from those of other opioids.
Adverse events considered to be at least possibly related to treatment were:
Frequent(may affect up to 1 in 10 people):
- Excessive fatigue/drowsiness, dizziness, headache, sedation.
- Vision problems (e.g., blurred or double vision).
- Nausea/feeling sick, constipation, vomiting, dry mouth.
- Itching of the skin.
- Fatigue.
- Confusion.
Uncommon(may affect up to 1 in 100 people):
- High blood pressure.
- Taste disturbances, inactivity, memory problems, thought disorders.
- Slow or shallow breathing, sinus congestion.
- Diarrhea, inflammation of the buccal mucosa, gum bleeding, indigestion, mouth ulcers, mouth pain, pain when swallowing.
- Involuntary loss of urine.
- Increased sweating, tendency to bruise.
- Muscle cramps, muscle weakness, joint pain, muscle pain, pain in the limbs, jaw pain.
- Decreased appetite.
- Accidental injuries (e.g., falls).
- Hot flashes/sensation of heat.
- Weakness, chills, fever, thirst.
- Feeling anxious or nervous, hallucinations, delirium, abnormal dreams, insomnia, restlessness.
Rare(may affect up to 1 in 10,000 people):
- Muscle spasms, convulsions, abnormal sensations such as tingling, numbness, increased sensitivity to touch also around the mouth, difficulty coordinating movements.
- Severe breathing difficulties.
- Abdominal pain, flatulence, abdominal distension.
- Difficulty urinating.
- Skin rash.
- Vasodilation.
- General feeling of discomfort.
- Inflammation of arms and legs.
- Abnormal thoughts, feeling of unreality, depression, mood changes, excessive feeling of well-being.
Frequency not known(cannot be estimated from the available data):
- Delirium (symptoms may include a combination of agitation, restlessness, disorientation, confusion, fear, seeing or hearing things that do not really exist, sleep disturbances, nightmares).
- Withdrawal syndrome (which may manifest with the following adverse effects: nausea, vomiting, diarrhea, anxiety, chills, tremors, and sweating).
- Pharmacological tolerance, drug dependence (addiction).
- Drug abuse (see section 2).
- Lack of male sex hormones (androgen deficiency).
There is a risk of abuse or addiction with Breakyl. The risk is greater if you have ever been addicted to or abused other medications, illegal drugs, or alcohol.
Prolonged treatment with fentanyl during pregnancy may cause withdrawal symptoms in the newborn, which can be potentially fatal (see section 2).
If you consider any of the side effects you are experiencing to be serious or if you notice any side effect not mentioned in this leaflet, inform your doctor or pharmacist.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency's (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Breakyl
Keep this medication in a safe and protected place, where others cannot access it. This medication can cause serious harm and even be fatal if used accidentally or intentionally by someone who has not been prescribed it.
Do not use Breakyl after the expiration date stated on the packaging and on each sachet, expressed as (MM. YYYY). The expiration date is the last day of the month indicated.
Do not store above 30°C.
Do not refrigerate.
Keep in the original packaging to protect it from moisture.
Do not use it if the sachet is damaged before opening. If the buccal film is damaged or cut while you are taking it out, it should not be used.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medication at the SIGRE collection point in your usual pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Breakyl composition
The active substance is fentanyl.
Breakyl 200 micrograms buccal film
One buccal film contains 200 micrograms of fentanyl (as fentanyl citrate),
Breakyl 400 micrograms buccal film
One buccal film contains 400 micrograms of fentanyl (as fentanyl citrate),
Breakyl 600 micrograms buccal film
One buccal film contains 600 micrograms of fentanyl (as fentanyl citrate),
Breakyl 800 micrograms buccal film
One buccal film contains 800 micrograms of fentanyl (as fentanyl citrate), or
Breakyl 1200 micrograms buccal film
One buccal film contains 1200 micrograms of fentanyl (as fentanyl citrate).
The other ingredients are:
Active layer:
Propylene glycol (E1520),
Sodium benzoate (E211),
Methyl parahydroxybenzoate (E218),
Propyl parahydroxybenzoate (E216),
Iron oxide (red) (E172),
Anhydrous citric acid,
All-rac-α-tocopherol acetate,
Anhydrous sodium dihydrogen phosphate,
Sodium hydroxide,
Anhydrous trisodium phosphate,
Polycarbophil,
Hydroxypropylcellulose,
Hydroxyethylcellulose,
Sodium carmellose.
Support layer:
Sodium benzoate (E211),
Methyl parahydroxybenzoate (E218),
Propyl parahydroxybenzoate (E216),
Anhydrous citric acid,
All-rac-α-tocopherol acetate,
Hydroxypropylcellulose,
Hydroxyethylcellulose,
Titanium dioxide (E171),
Sodium saccharin,
Peppermint oil.
This medication contains, depending on the concentration, a maximum of 0.69 mg of sodium benzoate per unit dose (see section 2) and less than 1 mmol of sodium (23 mg) per buccal film, i.e., it is essentially sodium-free.
Appearance of Breakyl and package contents
Breakyl is a soluble, rectangular, flat, and flexible buccal film, with one pink side and one white side, designed to release fentanyl directly into the bloodstream. The pink side contains the active substance fentanyl. The white side minimizes the release of fentanyl into the saliva to prevent the active substance from being swallowed.
The following template shows the size of the available Breakyl strengths:
micrograms micrograms micrograms micrograms micrograms
Each buccal film is individually packaged in a child-resistant sachet.
Breakyl is available in the following presentations:
Breakyl 200 micrograms: packages with 4, 10, or 28 sachets, each containing one buccal film.
Breakyl 400 micrograms: packages with 4, 10, or 28 sachets, each containing one buccal film.
Breakyl 600 micrograms: packages with 4, 10, or 28 sachets, each containing one buccal film.
Breakyl 800 micrograms: packages with 4, 10, or 28 sachets, each containing one buccal film.
Breakyl 1200 micrograms: packages with 4, 10, or 28 sachets, each containing one buccal film.
Not all pack sizes may be marketed.
Marketing Authorization Holder
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublin 15
Dublin
Ireland
Manufacturer
MEDA Pharma GmbH & Co. KG
Benzstr. 1
D-61352 Bad Homburg
Germany
Or
LTS Lohmann Therapie Systeme AG
Lohmannstrasse 2
D-56626 Andernach
Germany
You can request more information about this medication from the local representative of the Marketing Authorization Holder:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Germany, Austria, Bulgaria, Slovakia, Spain, France, Greece, Poland, Portugal, Czech Republic: Breakyl / Breakyl Start
Date of the last revision of this leaflet:May 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) https://www.aemps.gob.es/
- Country of registration
- Average pharmacy price201.43 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BREAKYL 1200 micrograms ORAL FILMDosage form: TRANSDERMAL PATCH, 12 MCG/HActive substance: fentanylManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1200 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: BUCCAL/SUCKING TABLET, 1600 microgramsActive substance: fentanylManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for BREAKYL 1200 micrograms ORAL FILM
Discuss questions about BREAKYL 1200 micrograms ORAL FILM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















