
BRALTUS 10 micrograms/dose released powder for inhalation (hard capsule)

How to use BRALTUS 10 micrograms/dose released powder for inhalation (hard capsule)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for thepatient
Braltus 10 micrograms/dosereleasedpowder for inhalation (hard capsule)
tiotropium
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again. If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Braltus and what is it used for
- What you need to know before starting to use Braltus
- How to use Braltus
- Possible side effects
- Storage of Braltus
- Package contents and additional information
1. What is Braltus and what is it used for
Braltus contains the active ingredient tiotropium. Tiotropium helps people with chronic obstructive pulmonary disease (COPD) to breathe more easily. Chronic obstructive pulmonary disease (COPD) is a chronic lung disease that causes difficulty breathing and coughing. The term COPD is associated with chronic bronchitis and emphysema. Since COPD is a chronic disease, you must use this medication every day and not just when you have breathing problems or other symptoms of COPD.
Braltus is a long-acting bronchodilator that helps to open the airways and facilitate the intake and expulsion of air from the lungs. Regular use of this medication can also help you when you have persistent difficulty breathing due to your disease and will help you minimize the effects of the disease on your daily life. It will also help you to be active for longer. Daily use of this medication will also help you prevent sudden and short-term symptoms of COPD worsening, which can last for several days. The effect of this medication lasts 24 hours, so you only need to use it once a day.
This medication should not be used as rescue therapy for the treatment of sudden chest tightness, coughing, wheezing, or shortness of breath. Please use a rapid-acting inhaler (rescue), such as salbutamol. Carry this rescue inhaler with you at all times.
2. What you need to know before starting to use Braltus
Do not use Braltus:
- if you are allergic to tiotropium or any of the other components of this medication (listed in section 6).
- if you are allergic to atropine or similar medications such as ipratropium or oxitropium
- if you are allergic to lactose or other sugars
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Braltus:
- if you are taking other medications that contain ipratropium or oxitropium
- if you have narrow-angle glaucoma, prostate problems, or difficulty urinating
- if you have any kidney problems
- if you have had a heart attack in the last 6 months, unstable or life-threatening irregular heartbeats, or severe heart failure in the last year.
Braltus is indicated for the maintenance treatment of your chronic obstructive pulmonary disease, it should not be used to treat a sudden episode of shortness of breath or wheezing (bronchospasm).
After administration of Braltus, immediate allergic reactions such as rash, swelling, itching, wheezing, or shortness of breath may occur. If this happens, please consult your doctor immediately.
Inhaled medications like Braltus may cause chest tightness, coughing, wheezing, or shortness of breath immediately after inhalation. If this happens, use a rapid-acting inhaler (rescue) immediately, such as salbutamol. If these symptoms appear, discontinue the use of Braltus and consult your doctor immediately.
Be careful that the inhalation powder does not enter your eyes, as this can cause watery eyes, or could cause or worsen narrow-angle glaucoma, which is an eye disease. Eye pain or discomfort, blurred vision, halos, or colored images associated with eye redness may be signs of an acute narrow-angle glaucoma episode. Eye symptoms may be accompanied by headache, nausea, or vomiting. You should discontinue the use of this medication and consult your doctor immediately, preferably an ophthalmologist, when these signs and symptoms of narrow-angle glaucoma appear.
Inhaled medications may decrease normal saliva secretion in your mouth and produce dry mouth. This may be associated with dental caries in the long term. Therefore, remember to take care of your oral hygiene, rinse your mouth, and brush your teeth regularly.
In case you have had a heart attack in the last 6 months, unstable or life-threatening irregular heartbeats, or severe heart failure in the last year, inform your doctor. This is important to decide if Braltus is the right medication for you.
You should not use this medication more than once a day (see section 3).
Children and adolescents
Braltus is not recommended for children and adolescents under 18 years of age.
Use of Braltus withother medications
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication, including any other inhaler and medications purchased without a prescription.
Tell your doctor or pharmacist if you are using or have used similar medications for your lung disease, such as ipratropium or oxitropium.
No specific adverse reactions have been reported when this medication has been used with other medications commonly used for the treatment of COPD, such as rescue inhalers, e.g., salbutamol, methylxanthines, such as theophylline, and/or oral and inhaled steroids, such as prednisolone.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. You should not use this medication unless your doctor has specifically recommended it.
Driving and using machines
The occurrence of dizziness, blurred vision, or headache may affect your ability to drive and use machines.
Braltus contains lactose
Lactose is a type of sugar found in cow's milk. If your doctor has told you that you have an intolerance to certain sugars, consult with them before using this medication. It may cause allergic reactions in patients with cow's milk protein allergy.
If administered according to the recommended dose, one capsule once a day, each dose provides up to 18 mg of lactose monohydrate.
3. How to use Braltus
Follow the instructions for administration of this medication indicated by your doctor or pharmacist exactly. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is the inhalation of the contents of onecapsule once a daywith the Zonda inhaler. One capsule provides the necessary daily dose of tiotropium (released dose of 10 micrograms of tiotropium). Do not use more than the recommended dose.
You should try to use this medication at the same time every day. This is important because this medication is effective for 24 hours.
The capsules are for inhalation only and not for oral ingestion.
Do not swallow the capsules.
The Zonda inhaler, in which you must insert the Braltus capsule, pierces the capsule and allows you to inhale the powder. The capsules should only be inhaled using the Zonda inhaler. Do not use other inhalers to administer the Braltus capsules.
Make sure you know how to use the Zonda inhaler correctly. The instructions for use of the inhaler are located behind this leaflet. Remember to follow these instructions carefully. Additionally, additional images are provided for the correct placement of the capsule in the inhaler on the inside of the package lid. To avoid the risk of asphyxiation, NEVERplace a capsule directly into the mouthpiece.If you have any problems using the Zonda inhaler, ask your doctor, nurse, or pharmacist to show you how it works.
If necessary, you can clean the mouthpiece of your Zonda inhaler after use with a dry paper towel.
Make sure you do not blow into the Zonda inhaler. When using Braltus, be careful and do not let the powder enter your eyes. If powder enters your eyes, it could cause blurred vision, pain, and/or eye redness. You should rinse your eyes immediatelywith warm water. Consult your doctor immediatelyfor more information.
If you notice that your breathing worsens, consult your doctor as soon as possible.
Use in children and adolescents
Braltus is not recommended for children and adolescents under 18 years of age.
If you use more Braltus than you should
If you inhale more than 1 Braltus capsule in a day, you should talk to your doctor immediately. You may have a higher risk of experiencing a side effect such as dry mouth, constipation, difficulty urinating, increased heart rate, or blurred vision.
If you forget to use Braltus
If you have forgotten to take a dose, do so as soon as you remember, but do nottake two doses at the same time or on the same day. Then, take your next dose as usual. Do not take a double dose to make up for forgotten doses.
If you stop treatment with Braltus
Before stopping treatment with Braltus, you should talk to your doctor or pharmacist. If you stop using this medication, the signs and symptoms of COPD may worsen.
If you have any other doubts about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
After using this medication, individual or severe allergic reactions (anaphylactic reaction) may occur, including reactions that cause swelling of the face and throat (angioedema) or other hypersensitivity reactions (such as a sudden drop in blood pressure or dizziness), or increased wheezing and shortness of breath. Additionally, as with all inhaled medications, some patients may experience unexpected chest tightness, coughing, wheezing, or shortness of breath immediately after inhalation (bronchospasm).
If you experience any of these reactions, consult your doctor immediately.
Do not use Braltus again until you see or at least talk to your doctor. If you have wheezing and shortness of breath, use your rapid-acting inhaler (rescue) immediately.
Other side effects have been experienced by people who have used this medication and are listed according to their frequency:
Common: may affect up to 1 in 10 people
- Dry mouth: this is usually mild
Uncommon: may affect up to 1 in 100 people
- dizziness
- headache
- alterations in taste
- blurred vision
- irregular heartbeat (atrial fibrillation)
- throat inflammation (pharyngitis)
- hoarseness (dysphonia)
- coughing
- heartburn (gastroesophageal reflux)
- constipation
- fungal infection in the mouth or throat (oropharyngeal candidiasis)
- rash
- difficulty urinating (urinary retention)
- painful urination (dysuria)
Rare: may affect up to 1 in 1,000 people
- difficulty sleeping (insomnia)
- halos or colored images associated with eye redness (glaucoma)
- increased eye pressure
- irregular heartbeat (supraventricular tachycardia)
- increased heart rate (tachycardia)
- palpitations
- chest tightness associated with coughing, wheezing, or shortness of breath immediately after inhalation (bronchospasm)
- nasal bleeding (epistaxis)
- larynx inflammation (laryngitis)
- sinus inflammation (sinusitis)
- intestinal blockage or absence of intestinal movement (intestinal obstruction including paralytic ileus)
- gum inflammation (gingivitis)
- tongue inflammation (glossitis)
- difficulty swallowing (dysphagia)
- mouth inflammation (stomatitis)
- feeling of dizziness (nausea)
- hypersensitivity, including immediate reactions
- severe allergic reaction that causes swelling of the face and throat (angioedema)
- hives
- itching (pruritus)
- urinary tract infection
Not known: cannot be estimated from the available data
- water loss (dehydration)
- dental caries
- skin infections or ulcers
- dry skin
- joint swelling
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Braltus
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date that appears on the box and label of the bottle after CAD or EXP. The expiration date is the last day of the month indicated.
Do not refrigerate or freeze.
Keep the bottle tightly closed. Store in the original packaging to protect it from moisture.
Use this medication within 30 days (15-capsule bottle) or 60 days (30-capsule bottle) after opening the bottle.
The Zonda inhaler can only be used with the capsule bottle that can be provided in the same package as the inhaler or packaged in a separate box from the inhaler. Do not reuse the inhaler for another capsule bottle.
Medications should not be thrown away through the sewers or in the trash. Deposit the packaging and medications you no longer need in the SIGRE Point of the pharmacy. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
The inhalation device should be disposed of according to local requirements.
6. Container Contents and Additional Information
Composition of Braltus 10 micrograms/delivered dose inhalation powder
- The active ingredient is tiotropium. Each capsule contains 13 micrograms of active ingredient tiotropium (as tiotropium bromide). During inhalation, 10 micrograms of tiotropium are released from the mouthpiece of the Zonda inhaler and inhaled into the lungs.
- The other ingredients are lactose monohydrate (capsule content) and hypromellose (capsule coating).
Appearance of the Product and Container Contents
Braltus 10 micrograms/delivered dose inhalation powder is a hard, colorless, and transparent capsule containing white powder.
This medication is presented in bottles with a screw cap. The bottle is presented in a box with the Zonda inhaler. The Zonda inhaler has a green body and cap with a white button.
Braltus is available in the following containers:
20 ml or 35 ml container with 15 capsules and a Zonda inhaler
35 ml container with 30 capsules and a Zonda inhaler
Multi-container with 60 capsules (2 containers of 30 capsules) and 2 Zonda inhalers
Multi-container with 90 capsules (3 containers of 30 capsules) and 3 Zonda inhalers
Packaged container: container with 30 capsules (bottle) packaged with another container with 1 Zonda inhaler separately.
Only some container sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Teva Pharma, S.L.U.
C/Anabel Segura, 11 Edificio Albatros B, 1ª Planta
Alcobendas, 28108 Madrid (Spain)
Manufacturer
Laboratorios LICONSA S.A.
Avda. Miralcampo, No 7, Polígono Industrial Miralcampo
19200 Azuqueca de Henares, Guadalajara
Spain
or
Teva Operations Poland Sp.z.o.o.
Ul. Mogilska 80
31-546 Krakow, Poland
or
Actavis Ltd
BLB015, BLB016, Bulebel Industrial Estate,
Zejtun, ZTN3000,
Malta
This medication is authorized in the Member States of the European Economic Area under the following names:
Austria: Braltus 10 Mikrogramm pro abgegebener Dosis Kapseln mit Inhalationspulver
Belgium: Braltus 10 micrograms Inhalatiepoeder in harde capsule/ Poudre pour inhalation en gélule/ Hartkapsel mit Pulver zur Inhalation
Bulgaria: Braltus 10 ?????????? ?? ????????? ???? ???? ?? ?????????, ?????? ???????
Cyprus: Braltus 10 μικρογραμμ?ρια αν? χορηγο?μενη δ?ση, κ?νις για εισπνο?, καψ?κιο, σκληρ?
Czech Republic: Braltus 10 mikrogramu/dávka, prášek k inhalaci ve tvrdých tobolkách
Germany: Braltus 10 Mikrogramm Hartkapseln mit Pulver zur Inhalation
Denmark: Braltus
Greece: Braltus 10 μικρογραμμ?ρια αν? χορηγο?μενη δ?ση, κ?νις για εισπνο?, καψ?κιο, σκληρ?
Spain: Braltus 10 microgramos/dosis liberada polvo para inhalación (cápsula dura)
Finland: Braltus 10 mikrog / vapautunut annos inhalaatiojauhe, kapseli, kova
Croatia: Braltus 10 mikrograma mikrograma po isporucenoj dozi, prašak inhalata, tvrde kapsule
Hungary: Braltus 10 mikrogramm adagolt inhalációs por kemény kapszulában
Ireland: Braltus 10 microgram per delivered dose inhalation powder, hard capsule
Italy: Tiotropio Teva Italia 10 microgrammi per dose erogata polvere per inalazione, capsula rigida
Luxembourg: Braltus 10 Mikrogramm pro abgegebener Dosis Kapsel mit Inhalationspulver
Netherlands: Tiotrus 10 microgram per afgegeven dosis inhalatiepoeder in harde capsules
Norway: Braltus
Poland: Braltus
Portugal: Braltus
Romania: Gregal 10 micrograme pulbere de inhalat, capsula
Sweden: Braltus
Slovakia: Braltus 10 mikrogramov
United Kingdom: Braltus 10 microgram per delivered dose inhalation powder, hard capsule
Date of the last revision of this leaflet: August 2018
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
<------------------------------------------------------------------------------------------------------------------?
Zonda Inhaler Instructions for Use
Dear patient,
The Zonda inhaler allows you to inhale the medication contained in the Braltus capsule prescribed by your doctor for your respiratory problems.
Remember to carefully follow your doctor's instructions for using Braltus. The Zonda inhaler is specifically designed for Braltus capsules, so it should not be used for any other medication.
The capsules should only be inhaled using the Zonda inhaler. Do notuse other inhalers to administer Braltus capsules. Each capsule contains only a small amount of powder.
Do notopen the capsule or it may not work.
The Zonda inhaler should be used with the bottle of capsules provided in the same container or in a separate container packaged with another container with the inhaler. Do not reuse the inhaler for any other bottle of capsules. Dispose of the Zonda device after 15 uses (if you have used the 15-capsule bottle) or 30 uses (if you have used the 30-capsule bottle).
Zonda

- Protective cap
- Mouthpiece
- Base
- Piercing button
- Central chamber
- Pull the protective cap upwards

- Hold the base of the inhaler firmly. Then, open the mouthpiece by lifting it upwards in the direction of the arrow.

- Remove a Braltus capsule from the bottle immediately before use and close the bottle perfectly. Place a capsule in the central chamber at the base of the inhaler. Do not store the capsule in the Zonda inhaler.
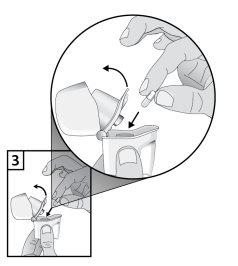
- To avoid the risk of choking, NEVER place a capsule directly inside the mouthpiece.
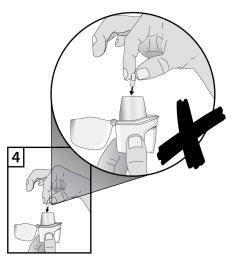
- Close the mouthpiece firmly until you hear a click, leaving the protective cap open.
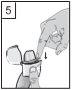
- Hold the inhaler with the mouthpiece upwards, press the piercing button all the way down once, and release it. This action pierces the capsule and allows the medication to be released when inhaled.
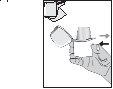
- Exhale deeply. Important: never exhale into the mouthpiece.

- Bring the inhaler to your mouth and keep your head in an upright position. Close your lips tightly around the mouthpiece and inhale slowly and deeply, but sufficiently to hear or feel the capsule vibrate inside the central chamber. Hold your breath for as long as you feel comfortable while removing the inhaler from your mouth. Continue breathing normally. Repeat steps 7 and 8 once more; this will completely empty the capsule.
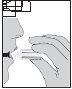
- After use, open the mouthpiece again and discard the empty capsule. Close the mouthpiece and protective cap to store the Zonda inhaler.
The Zonda inhaler is a Medical Device (CE)
Manufacturer:
Laboratorios LICONSA S.A.
Avda. Miralcampo, No 7, Polígono Industrial Miralcampo
19200 Azuqueca de Henares, Guadalajara
Spain

0051
- Country of registration
- Average pharmacy price39.25 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRALTUS 10 micrograms/dose released powder for inhalation (hard capsule)Dosage form: PULMONARY INHALATION, 10 microgramsActive substance: tiotropium bromideManufacturer: Teva Pharma S.L.U.Prescription requiredDosage form: PULMONARY INHALATION, 18 microgramsActive substance: tiotropium bromideManufacturer: Boehringer Ingelheim International GmbhPrescription requiredDosage form: PULMONARY INHALATION, 2.5 micrograms / sprayActive substance: tiotropium bromideManufacturer: Boehringer Ingelheim International GmbhPrescription required
Online doctors for BRALTUS 10 micrograms/dose released powder for inhalation (hard capsule)
Discuss questions about BRALTUS 10 micrograms/dose released powder for inhalation (hard capsule), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















