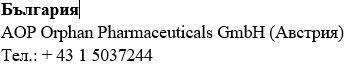BESREMI 250 micrograms/0.5 ml solution for injection in a prefilled pen

How to use BESREMI 250 micrograms/0.5 ml solution for injection in a prefilled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Besremi 250micrograms/0.5ml solution for injectionin a pre-filled pen
ropeginterferon alfa-2b
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Besremi and what is it used for
- What you need to know before you use Besremi
- How to use Besremi
- Possible side effects
- Storage of Besremi
- Contents of the pack and other information
1. What is Besremi and what is it used for
Besremi contains the active substance ropeginterferon alfa-2b, which belongs to a class of medicines known as interferons. Your immune system produces interferons to block the growth of cancer cells.
Besremi is used as monotherapy for the treatment of polycythaemia vera in adults. Polycythaemia vera is a type of cancer in which the bone marrow produces too many red blood cells, white blood cells, and platelets (the cells that help the blood to clot).
2. What you need to know before you use Besremi
Do not useBesremi
- if you are allergic to ropeginterferon alfa-2b or any of the other ingredients of this medicine (listed in section 6).
- if you have an uncontrolled thyroid disease.
- if you have had severe mental health problems (such as depression or suicidal thoughts, or if you have attempted suicide).
- if you have had severe heart problems recently (such as a heart attack or stroke).
- if you have or have had an autoimmune disease (such as rheumatoid arthritis, psoriasis, or inflammatory bowel disease).
- if you have received an organ transplant and are taking medicines to suppress your immune system.
- if you are taking telbivudine (a medicine used to treat hepatitis B infection).
- if you have advanced and uncontrolled liver disease.
- if you have severe kidney disease (which makes your kidneys work less than 15% of normal).
Warnings and precautions
Talk to your doctor before you start using Besremi:
- if you have a thyroid disease.
- if you have diabetes or high blood pressure; your doctor may ask you to have an eye examination.
- if you have liver problems, you will have regular blood tests to check your liver function if you have been taking Besremi for a long time.
- if you have kidney problems.
- if you have psoriasis or other skin problems, as they may get worse during treatment with Besremi.
Once you have started treatment with Besremi, talk to your doctor:
- if you develop symptoms of depression (such as feeling sad, depressed, and having suicidal thoughts).
- if you develop signs of a severe allergic reaction (such as difficulty breathing, wheezing, or hives); in this case, you should seek medical help immediately.
- if you develop symptoms of a cold or other respiratory infection (such as difficulty breathing, cough, fever, and chest pain).
- if you have changes in your vision, you should consult your doctor, who will have your eyes examined immediately. Serious eye problems can occur during treatment with Besremi. Normally, your doctor will have your eyes examined before you start treatment. If you have health problems that can cause eye problems, such as diabetes or high blood pressure, your doctor will also have your eyes examined during treatment. If your vision gets worse, your doctor may decide to stop your treatment.
Medicines based on interferons can also cause dental and gum disorders, with the possibility of tooth loss. Additionally, dry mouth can be harmful to teeth or mouth mucosa during long-term treatment with Besremi. You should brush your teeth well twice a day and have regular dental check-ups.
It will take some time to reach your optimal individual dose of Besremi. Your doctor will decide if you need to be treated with another medicine to quickly reduce the number of cells in your blood, in order to prevent the formation of clots and bleeding.
Children and adolescents
Do not give this medicine to children or adolescents, as there is no information available on the use of Besremi in this age group.
Other medicines and Besremi
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Do not use Besremi if you are taking telbivudine (for the treatment of hepatitis B), as the combination of these medicines increases the risk of developing peripheral neuropathy (numbness, tingling, or burning sensation in the arms and legs). Tell your doctor if you are receiving treatment with telbivudine.
Particularly tell your doctor if you are taking any of the following medicines:
- theophylline (a medicine used to treat respiratory diseases such as asthma)
- methadone (a medicine used to treat pain or opioid dependence)
- vortioxetine or risperidone (medicines used to treat mental disorders)
- cancer medicines such as those that stop or slow down the growth of blood cells in the bone marrow (e.g. hydroxyurea)
- medicines that act on the central nervous system to relieve pain, help you sleep, or calm you down (e.g. morphine, midazolam)
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
The effect of Besremi during pregnancy is unknown. The use of Besremi is not recommended during pregnancy. If you are able to become pregnant, your doctor will explain which effective contraceptive method you should use during your treatment with Besremi.
Breast-feeding
It is unknown whether Besremi passes into breast milk. Your doctor will help you decide whether to stop breast-feeding while using this medicine.
Driving and using machines
Do not drive or use machines if you feel dizzy, drowsy, or confused while using Besremi.
Besremi contains benzyl alcohol
This medicine contains 5 mg of benzyl alcohol per 0.5 ml. Benzyl alcohol may cause allergic reactions.
Talk to your doctor or pharmacist:
- if you are pregnant or breast-feeding.
- if you have liver or kidney disease,
This is because large amounts of benzyl alcohol can accumulate in the body and cause side effects (metabolic acidosis).
Besremi contains polysorbate 80
This medicine contains 0.025 mg of polysorbate 80 in each 0.5 ml. Polysorbates can cause allergic reactions. Tell your doctor if you have any known allergies.
Besremi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per ml; this is essentially “sodium-free”.
3. How to use Besremi
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
Your doctor will determine the dose you need individually for your disease. The usual starting dose of Besremi is 100 micrograms every 2 weeks. After that, your doctor will gradually increase the dose and may change it during treatment. If you have severe kidney problems, the starting dose given by your doctor will be reduced to 50 micrograms.
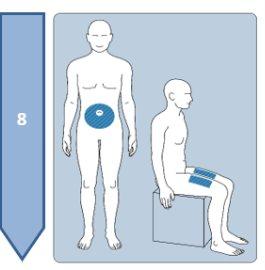
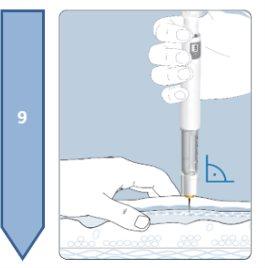
This medicine is given by subcutaneous injection, which means it is injected into the tissue under the skin. It must not be injected into irritated skin, red skin, skin with bruises, infected skin, or skin with scars.
If you inject this medicine yourself, you will be taught how to prepare the medicine and inject it.
Never share the pre-filled pen Besremi with anyone, even if you change the needle, in order to avoid infectious diseases.
You can find the details of the preparation and injection of Besremi in the instruction manual. Read them before you start using Besremi.
If you use more Besremi than you should
Talk to your doctor as soon as possible.
If you forget to use Besremi
The dose should be injected as soon as you remember. But if it has been more than 2 days since you missed the dose, skip it and inject the next dose when it is due. Do not inject a double dose to make up for the missed doses. If you are not sure, ask your doctor or pharmacist again.
If you stop using Besremi
Do not stop using Besremi without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following serious side effects during your treatment with Besremi, contact your doctor immediately:
Common side effects (may affect up to 1 in 10 people):
- changes in heart rhythm (when the heart beats too fast or irregularly)
Uncommon side effects (may affect up to 1 in 100 people):
- attempted suicide, thoughts of suicide
- loss of vision, which can be due to a hemorrhage in the retina (the retina is the layer of the eye that is sensitive to light) or to the accumulation of fat in the retina or under it.
Rare side effects (may affect up to 1 in 1,000 people):
- loss of vision, which can be due to an injury to the retina (such as blockage of the blood vessels of the eye) or to the optic nerve.
Very rare side effects (may affect up to 1 in 10,000 people):
- blindness
- respiratory problems such as difficulty breathing, cough, and chest pain, which can be due to pulmonary infiltration, pneumonia (lung infection), pulmonary arterial hypertension (increase in blood pressure in the vessels that carry blood from the heart to the lungs), and pulmonary fibrosis (lung disease in which scars are formed in the lung tissue)
Frequency not known (cannot be estimated from the available data):
- retinal detachment (can cause problems in the eyes and vision)
Other side effects
Very common side effects (may affect more than 1 in 10 people):
- decrease in the number of a type of white blood cell (called leukocyte) and of the cells involved in blood clotting (called platelets)
- joint or muscle pain
- flu-like symptoms, feeling of tiredness
- in blood tests: increase in an enzyme called gamma-glutamyltransferase
Common side effects (may affect up to 1 in 10 people):
- respiratory tract infection, runny or blocked nose, fungal infections, flu
- reduction in the number or size of red blood cells
- increase or decrease in thyroid activity, increase in thyroid-stimulating hormone, thyroid inflammation
- increase in triglycerides (a type of lipid) in the blood, decrease in appetite
- aggressive behavior, feeling of depression, feeling of anxiety, problems sleeping or sleeping without interruption, mood changes, lack of energy or motivation
- headache, feeling of dizziness, reduction in touch or sensitivity, feeling of sleepiness, feeling of tingling and paresthesia
- dry eyes
- lesion of the small blood vessels of the body (capillaries)
- respiratory problems
- diarrhea, nausea, abdominal pain or digestive discomfort, constipation, dry mouth
- liver disorder, increase in certain liver enzymes (in blood tests)
- itching, hair loss, rash, redness of the skin, dry and scaly skin, acne, thickening of the outer layer of the skin, increased sweating
- Sjögren's syndrome (a disorder in which the immune system attacks the glands that produce fluids, such as tears and saliva), arthritis, pain in arms and legs, bone pain, sudden and painful muscle stiffness
- fever, weakness, chills, general health problems, irritation or redness at the injection site, weight loss
- in blood tests: antibodies produced by the immune system against red blood cells, increase in an enzyme called lactate dehydrogenase
Uncommon side effects (may affect up to 1 in 100 people):
- herpes infection and reinfection, bacterial infections
- increase in the number of platelets
- autoimmune thyroid disorder, sarcoidosis (areas of inflamed tissue in different parts of the body)
- diabetes
- panic attack, hallucination (seeing, hearing, or feeling things that are not there), feeling of stress, feeling of nervousness, loss of interest in activities, nightmares, irritability, confusion
- lesion of the nervous system, migraine, mental disorder (a health problem that affects thought, emotion, or behavior), visual or sensory disorders, dryness in the hands
- eye discomfort, eczema on the eyelids
- hearing loss, ringing in the ears (tinnitus), feeling of spinning (vertigo)
- heart disorders such as heart block (a disorder of the electrical activity of the heart), blood clots in the blood vessels of the heart, aortic valve insufficiency
- high blood pressure, reduced blood supply to certain parts of the body, hematoma (accumulation of blood under the skin), hot flashes
- inflammation of the lung tissue, cough, nosebleeds, sore throat
- inflammation of the stomach, disorder of the abdominal wall, gas in the intestines, indigestion, pain when swallowing, bleeding gums
- inflammation of the liver, liver damage, increase in liver size
- sensitivity to sunlight, skin peeling, nail disorders
- muscle weakness, neck pain and groin pain
- inflammation of the bladder, painful urination, increased need to urinate, inability to urinate
- sexual problems
- pain or itching at the injection site, sensitivity to weather changes
- porphyria (a liver disorder in which substances called porphyrins accumulate in the skin, causing local damage to the skin, such as rashes, blisters, sores, or discomfort, when exposed to sunlight)
- in tests: increase in uric acid, antibodies produced by the immune system against red blood cells
Rare side effects (may affect up to 1 in 1,000 people):
- bipolar disorders (mood disorders with episodes of sadness and nervousness), mania (excessive excitement or unreasonable enthusiasm)
- myocardial disease (diseases that affect the heart muscle), angina pectoris (severe chest pain due to blockage of the heart vessels)
- liver failure
Very rare side effects (may affect up to 1 in 10,000 people):
- thrombotic thrombocytopenic purpura or idiopathic thrombocytopenic purpura (increase in bruising and bleeding, decrease in the number of platelets, anemia, and extreme weakness)
- myocardial ischemia (reduced blood flow to the heart muscle)
Frequency not known (cannot be estimated from the available data):
- Vogt-Koyanagi-Harada disease (a rare disease that can cause loss of vision, hearing, and skin pigmentation), severe allergic reaction
- skin color change
- periodontal and dental disorders, tongue color change
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Besremi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the carton after “EXP”. The expiry date is the last day of the month shown.
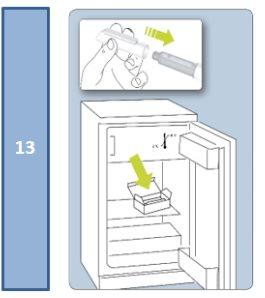
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep the pre-filled pen in its outer packaging to protect it from light.
Once opened, the pre-filled pen can be stored in the refrigerator (between 2°C and 8°C) for a maximum of 30 days, if stored with the cap on and in the carton, which protects it from light.
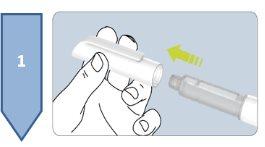
Do not use this medicine if you notice that the pre-filled pen is damaged, if the solution is not clear, if it has particles or flakes, if it is not colorless, or if it has a different color than light yellow.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help to protect the environment.
6. Package Contents and Additional Information
Besremi Composition
- The active ingredient is ropeginterferon alfa-2b.
Each pre-filled pen with 0.5 ml of solution contains 250 micrograms of ropeginterferon alfa-2b expressed in protein, which corresponds to 500 micrograms/ml.
- The other components are sodium chloride, polysorbate 80, benzyl alcohol, anhydrous sodium acetate, glacial acetic acid, and water for injectable preparations. Regarding benzyl alcohol, polysorbate 80, and sodium, see section 2 "Besremi contains benzyl alcohol", "Besremi contains polysorbate 80", and "Besremi contains sodium".
Appearance of the Product and Package Contents
Besremi is presented in a solution for injection (injection) in a pre-filled pen. Each pre-filled pen contains 0.5 ml of solution. It is marketed in packages with:
- 1 pre-filled pen and 2 injection needles (type: mylife AutoProtect PRO 29G × 8 mm)
- 3 pre-filled pens and 6 injection needles (type: mylife AutoProtect PRO 29G × 8 mm).
Marketing Authorization Holder and Manufacturer
AOP Orphan Pharmaceuticals GmbH
Leopold-Ungar-Platz 2
1190 Vienna
Austria
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 | Lietuva AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
| Luxembourg/Luxemburg AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Ceská republika AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 | Magyarország AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Danmark AOP Orphan Pharmaceuticals GmbH (Austria) Tlf: +43 1 5037244 | Malta AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Deutschland AOP Orphan Pharmaceuticals Germany GmbH Tel: +49 89 99 740 7600 | Nederland AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Eesti AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 | Norge AOP Orphan Pharmaceuticals GmbH (Austria) Tlf: +43 1 5037244 |
Ελλάδα AOP Orphan Pharmaceuticals GmbH (Αυστρία) Τηλ: +43 1 5037244 | Österreich AOP Orphan Pharmaceuticals GmbH Tel: +43 1 5037244 |
España AOP Orphan Pharmaceuticals Iberia S.L.U. Tel: +34 91 449 19 89 | Polska AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
France AOP Orphan Pharmaceuticals GmbH (Austria) Tél: +43 1 5037244 | Portugal AOP Orphan Pharmaceuticals Iberia S.L.U. Tel: +34 91 449 19 89 |
Hrvatska AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 | România AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Ireland AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 | Slovenija AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Ísland AOP Orphan Pharmaceuticals GmbH (Austria) Sími: +43 1 5037244 | Slovenská republika AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Italia AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 | Suomi/Finland AOP Orphan Pharmaceuticals GmbH (Austria) Puh/Tel: +43 1 5037244 |
Κύπρος AOP Orphan Pharmaceuticals GmbH (Αυστρία) Τηλ: +43 1 5037244 | Sverige AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Latvija AOP Orphan Pharmaceuticals GmbH (Austria) Tel: +43 1 5037244 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
Instructions
Read this prospectus carefully before using the pre-filled pen Besremi 250 micrograms. If you have any doubts, consult your doctor or pharmacist.
Your doctor or pharmacist will teach you how to use the pen.
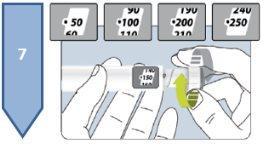
The pre-filled pen Besremi 250 micrograms can be used to inject doses within the range of 50 to 250 micrograms. The same pen can be used twice, as long as the sum of the two doses does not exceed 250 micrograms. Your doctor will indicate the dose you need. Take note of the dates and doses of the injections that your doctor indicates.
If you need a dose of more than 250 micrograms, you will need to use two pre-filled pens Besremi 250 micrograms. Each one should be injected at a different point. Your doctor or pharmacist will explain how to use the two pens.
Keep the pen in the outer packaging in the refrigerator.
Remove the pen from the refrigerator 15 minutes before injection to allow it to reach room temperature.
Sit in a well-lit and quiet area to administer the injection.
To administer the injection, you will need:
- Besremi pre-filled pen
- Needle (type: mylife AutoProtect PRO 29G × 8 mm)
- Wet wipe with alcohol (not included)
- Optional: adhesive plaster (not included)
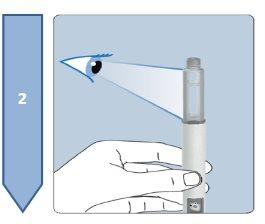
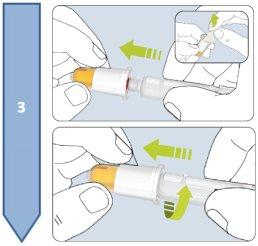
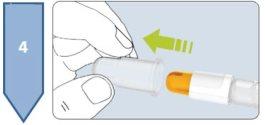
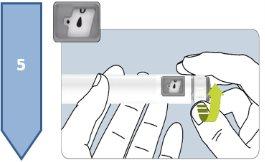
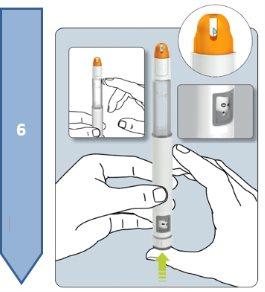
The Besremi pre-filled pen is supplied with two or six needles (depending on the package size). You must always use a new needle each time you inject.
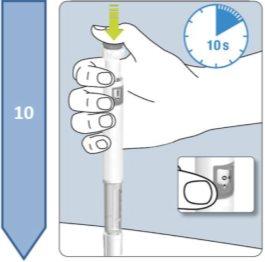
Do not use the pen if it appears damaged. If you feel that you may have damaged the pen while using it (e.g., it has fallen or has been pressed too hard), do not continue using it. Get a new one and start again.
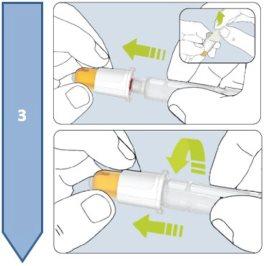
Description of the Besremi 250 micrograms pre-filled pen
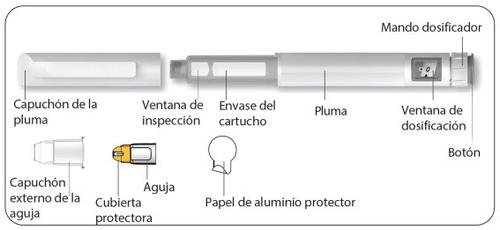
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
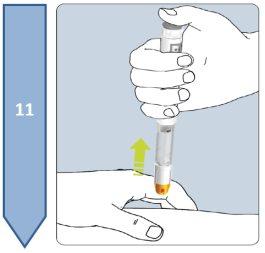
Do not inject into areas of irritated skin, redness, bruising, infection, or scarring. |
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
|
--------------------------------------------------------------------------------------------------------------------
|
Note:
|
--------------------------------------------------------------------------------------------------------------------
|
Note:
|
--------------------------------------------------------------------------------------------------------------------
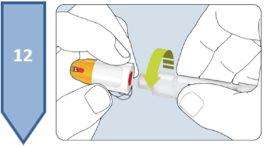
| Note:Put the pen cap back on. Reusing the Pen:
Disposal of the Pen and Needle:
|
--------------------------------------------------------------------------------------------------------------------
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BESREMI 250 micrograms/0.5 ml solution for injection in a prefilled penDosage form: INJECTABLE, 30 µgActive substance: interferon beta-1aManufacturer: Biogen Netherlands B.V.Prescription requiredDosage form: INJECTABLE, 30 µgActive substance: interferon beta-1aManufacturer: Biogen Netherlands B.V.Prescription requiredDosage form: INJECTABLE, 300 µgActive substance: interferon beta-1bManufacturer: Bayer AgPrescription required
Online doctors for BESREMI 250 micrograms/0.5 ml solution for injection in a prefilled pen
Discuss questions about BESREMI 250 micrograms/0.5 ml solution for injection in a prefilled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions