AVONEX 30 micrograms/0.5 ml INJECTABLE SOLUTION


How to use AVONEX 30 micrograms/0.5 ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
AVONEX 30 micrograms/0.5 ml solution for injection
(interferon beta-1a)
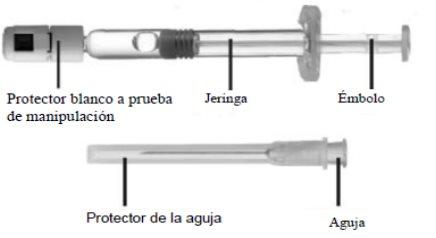
Pre-filled syringe
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Even if you have used Avonex before, some of the information may have changed.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
(Information notes) This leaflet is updated from time to time. Check for updates to this leaflet each time you renew your prescription. If you have any questions, ask your doctor or pharmacist. |
Contents of the pack
- What is AVONEX and what is it used for
- What you need to know before you use AVONEX
- How to use AVONEX
- Possible side effects
- Storing AVONEX
- Contents of the pack and further information
- How to inject AVONEX
1. What is AVONEX and what is it used for
What is AVONEX?
The active substance of Avonex is a protein called interferon beta-1a.Interferons are natural substances produced by your body to protect you from infections and diseases. The protein in Avonex is produced with exactly the same ingredients as the interferon beta present in the human body.
What is AVONEX used for?
Avonex is used to treat multiple sclerosis (MS).Treatment with Avonex may help prevent it from getting worse, although it will not cure MS.
Each patient has specific symptoms of MS.These can be:
- Feeling of instability or dizziness, walking problems, muscle stiffness and spasms, fatigue, tingling in the face, arms or legs
- Acute or chronic pain, bladder and bowel problems, sexual problems and vision problems
- Difficulty thinking and concentrating, depression.
MS also tends to flare up from time to time: this is what is known as a relapse.
(Information notes) Avonex works best when you use it:
Do not stop using Avonex without talking to your doctor. |
Avonex may help reduce the number of relapses you may have and slow down the disabling effects of MS.Your doctor will explain how long you can use Avonex or when to stop treatment.
How does AVONEX work?
Multiple sclerosis is associated with nerve damage (in the brain or spinal cord). In MS, your body's defense system reacts to your own myelin, i.e. the insulation that surrounds nerve fibers. When myelin is damaged, messages between the brain and other parts of the body are disrupted. This is what causes the symptoms of MS. Avonex appears to work by preventing your body's defense system from attacking myelin.
2. What you need to know before you use AVONEX
Do not use AVONEX
- if you are allergicto interferon beta or any of the other ingredients of this medicine (listed in section 6);
- if you have severe depressionor have had thoughts of suicide.
Talk to your doctor immediately in any of these cases.
(Information notes) Avonex and allergic reactions.As Avonex is made from a protein, there is a small chance that an allergic reaction may occur. Additional information on depression. You must not use Avonex if you have severe depression or have had thoughts of suicide. If you have depression, your doctor may prescribe Avonex, but it is essential that they know if you have had depression or any other problem that affects your mood. |
Warnings and precautions
Talk to your doctor before you start using Avonex if you have or have had in the past:
- Depressionor mood problems
- Suicidal thoughts.
You must tell your doctor immediately about changes in your mood, suicidal thoughts, unusual feelings of sadness, anxiety or hopelessness.
- Epilepsyor other uncontrolled seizure disorders
- Severe kidney or liver problems
- Low white blood cell or platelet counts, which may increase the risk of infection, bleeding or anemia
- Heart problemsthat may cause symptoms such as chest pain (angina),especially after any activity; swelling of ankles, difficulty breathing (congestive heart failure)or an irregular heartbeat (arrhythmias).
- Irritation at the injection site that can cause skin and tissue damage (injection site necrosis). When you are ready to administer the injection, carefully follow the instructions in section 7, "How to inject AVONEX" at the end of this leaflet. This reduces the risk of injection site reactions.
Talk to your doctor if you have any of these conditionsor if they get worse while using Avonex.
During treatment, blood clots may form in small blood vessels. These clots could affect your kidneys. This can happen after several weeks or several years after starting treatment with Avonex.
Your doctor may want to check your blood pressure, blood (platelet count), and kidney function.
Tell your doctor that you are using Avonex:
- If you are going to have a blood test.Avonex may interfere with the results.
(Information notes) There may be times when you need to remind other healthcare professionals that you are being treated with Avonex.For example, if you are prescribed other medicines or if you have a blood test, Avonex may interact with other medicines or with the test results. |
Pediatric population
Avonex is not recommended for use in children and adolescents because the data on the use of Avonex in this population are limited. Avonex must not be used in children under 10 years of age because it has not been established whether it would work for them and whether it would be safe.
Other medicines and AVONEX
Tell your doctorif you are using, have recently used, or might use any other medicines, especially for the treatment of epilepsy or depression. Avonex may affect other medicines or be affected by them. This includes any other medicine, including medicines obtained without a prescription.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
No harmful effects on the baby are expected. Avonex can be used during breastfeeding.
Driving and using machines
If you feel dizzy, do not drive.Avonex can make some people feel dizzy. If this happens to you or if you have any other side effect that may affect your ability, do not drive or use machines.
Important information about some of the ingredients of AVONEX:
This medicine is essentially sodium-free. It contains less than 23 mg (1 mmol) of sodium per weekly dose.
3. How to use AVONEX Dosage
Recommended weekly dose
One Avonex injection once a week.
Try to use Avonex at the same time on the same day each week.
If you have decided to start treatment with Avonex, your doctor may provide you with a dose adjustment device called Avostartclip. The Avostartclip attaches to the syringe and allows you to gradually increase the dose of Avonex when you start treatment. This is to limit the flu-like symptoms that some patients experience when they start using Avonex. Your doctor or nurse will help you use the Avostartclip dose adjustment device.
(Information notes) When starting Avonex If you are going to use Avonex for the first time, your doctor may advise you to start gradually increasing the dose to adjust to the effects of Avonex before administering the full dose. You will be provided with an Avostartclip dose adjustment device. The Avostartclip attaches to the syringe to administer a reduced dose of Avonex when you start treatment. Each Avostartclip is for single use and must be discarded along with the rest of Avonex. For details on its use, talk to your doctor. |
Self-injection
You can inject Avonex without your doctor's help if you have been taught how to do it. See the self-injection instructions at the end of this leaflet (see section 7, How to inject AVONEX).
If you have problemshandling the syringe, talk to your doctor. You may receive help.
(Information notes) More details on how to inject Avonex are included at the end of this leaflet. Alternative needle: The Avonex pack already contains an injection needle. Your doctor may prescribe a shorter and thinner needle, depending on your body characteristics. Talk to your doctor to find out if it is suitable for you. If you have problems handling the syringe, talk to your doctor about using a syringe holder. It is a specially designed support that helps you inject Avonex. |
Duration of treatment with AVONEX
Your doctor will tell you how long you should use Avonex. It is essential that you use Avonex regularly. Do not make any changes that your doctor has not told you to make.
If you inject more AVONEX than you should
You should only have one Avonex injection once a week. If you have had more than one Avonex injection within three days, contact your doctor or pharmacist immediately.
If you miss an injection
If you miss your usual weekly dose, inject a dose as soon as possible. Then skip the treatment for one week. Continue with the injection on that day of each week. If you have a preferred day for using Avonex, talk to your doctor to adjust the dose and get back to your preferred day. Do not give yourself two injections to make up for the missed injection.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
(Informative notes) Although the list of possible adverse effects may seem worrying, it is possible that you will not experience any of them. |
Severe Adverse Effects: Seek Medical Help
Severe Allergic Reactions
If you experience any of the following symptoms:
- Swelling of the face, lips, or tongue
- Difficulty breathing
- Rash.
Call a doctor immediately. Do not use Avonex until you have spoken with a doctor.
Depression
If you experience any symptoms of depression:
- Unusual feeling of sadness, anxiety, or hopelessness.
Call a doctor immediately.
Liver Problems
If you experience any of the following symptoms:
- Yellowing of the skin or the white part of the eyes (jaundice)
- Generalized itching
- Feeling of discomfort (nausea and vomiting)
- Bruises that appear easily on the skin.
Call a doctor immediatelyas they may be manifestations of a possible liver problem.
Adverse Effects Observed in Clinical Trials
(Informative notes) Adverse effects observed in clinical trials. These are adverse effects reported by people during the evaluation of Avonex. The figures are based on the number of people who experienced them. They give an idea of the likelihood that you will develop similar adverse effects. |
Very Common Adverse Effects
(may affect more than 1 in 10 people)
- Pseudoflu-like symptoms: headache, muscle pain, chills, or fever: see Pseudoflu-like symptoms, below
- Headache.
Common Adverse Effects
(may affect up to 1 in 10 people)
- Loss of appetite
- Feeling of weakness and fatigue
- Difficulty sleeping
- Depression
- Facial flushing
- Nasal discharge
- Diarrhea (soft stools)
- Feeling of discomfort (nausea or vomiting)
- Numbness or tingling of the skin
- Rash, skin bruising
- Increased sweating, night sweats
- Pain in muscles, joints, arms, legs, or neck
- Muscle cramps, stiffness in muscles and joints
- Pain, bruising, and redness at the injection site
- Changes in blood tests. The symptoms you may experience are fatigue, repeated infections, bruising, or bleeding without apparent cause.
Uncommon Adverse Effects
(may affect up to 1 in 100 people)
- Hair loss
- Changes in menstruation
- Burning sensation at the injection site.
Rare Adverse Effects
(may affect up to 1 in 1,000 people)
- Difficulty breathing
- Kidney problems, including scarring that can reduce kidney function. If you experience any of the following symptoms:
- Foamy urine
- Fatigue
- Swelling, especially of ankles and eyelids, and weight gain.
Report to your doctor as they may be manifestations of a possible kidney problem.
- Blood clots in small blood vessels that can affect your kidneys (thrombotic thrombocytopenic purpura or hemolytic uremic syndrome). The symptoms may include an increase in bruising, bleeding, fever, extreme weakness, headache, dizziness, or fainting. Your doctor may find changes in your blood and kidney function.
If you are concerned about any of the effects, talk to your doctor.
Other Adverse Effects
(Informative notes) These effects have been observed in people using Avonex, but we do not know how frequently they occur. If you feel dizzy, do not drive. |
- Thyroid dysfunction or excess activity
- Nervousness or anxiety, emotional instability, irrational thoughts or hallucinations (seeing or hearing things that are not real), confusion, or suicide
- Numbness, dizziness, seizures, or epileptic fits and migraines
- Perception of your heartbeat (palpitations), rapid or irregular heartbeat, or heart problems accompanied by the following symptoms: reduced exercise capacity, inability to stay lying down, difficulty breathing, or swelling of ankles
- Liver problems as described previously
- Hives or pseudovesicular rash, itching, worsening of existing psoriasis
- Swelling or bleeding at the injection site, tissue destruction (necrosis), or chest pain after an injection
- Weight gain or loss
- Changes in test results, such as variations in liver function tests
- Pulmonary arterial hypertension: a disease in which there is a significant narrowing of the blood vessels in the lungs, causing an increase in pressure in the blood vessels that carry blood from the heart to the lungs. Pulmonary arterial hypertension was observed at different times, even several years after starting treatment with interferon beta-containing medications.
If you are concerned about any of the effects, talk to your doctor.
Injection Site Effects
- Fainting sensation:The first injection of Avonex may be administered by your doctor. You may feel dizzy. You may even faint. It is unlikely to happen again.
- Immediately after the injection, you may notice your muscles are tense or very weak,as if you were going to repeat the experience. It is rare. It only occurs when self-injecting, and the effects pass quickly. They can occur at any time after starting treatment with Avonex.
- If you notice any skin irritation or other skin problemsafter an injection, talk to your doctor.
Pseudoflu-like Symptoms
(Informative notes) Three simple ways to reduce the impact of pseudoflu-like symptoms:
|
Some people report that after injecting Avonex, they experience a pseudoflu-like sensation.
The signs are:
- Headache
- Muscle pain
- Chills or fever.
These symptoms do not actually correspond to the flu
You cannot transmit them to anyone. They are more frequent when using Avonex for the first time. Your doctor may provide you with a dose adjustment device, Avostartclip, which will allow you to gradually increase the dose when starting treatment to help limit pseudoflu-like symptoms. Pseudoflu-like symptoms gradually decrease as more injections are administered.
Children (10 years of age or older) and adolescents
In clinical trials, some adverse effects were reported more frequently in children and adolescents than in adults, e.g., muscle pain, pain in a limb, fatigue, and joint pain.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaRAM.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
In order to improve the traceability of this medicine, your doctor or pharmacist must record the name and batch number of the medicine administered to you in your medical history. You may also want to take note of this information in case you are asked for it in the future.
5. Storage of AVONEX
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the label.
Store in the original packaging (sealed plastic tray) to protect it from light.
Store in the refrigerator (between 2°C and 8°C). Do not freeze.
Avonex can also be stored at room temperature (between 15°C and 30°C) for a maximum period of one week.
DO NOT use Avonexif you notice:
- The pre-filled syringe is broken.
- The sealed plastic tray is damaged or open.
- The solution is colored or you notice particles in suspension.
- The tamper-evident cap is damaged.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Contents and Additional Information
AVONEX Composition
The active ingredientis: interferon beta-1a 30 micrograms/0.5 ml.
The other componentsare: sodium acetate trihydrate, glacial acetic acid, arginine hydrochloride, polysorbate 20, and water for injectables.
Product Appearance and Container Contents
Avonex injectable is presented as a ready-to-use injection
A box of Avonex contains four or twelve pre-filled syringes, each with 0.5 ml of a clear and colorless liquid inside. Only certain package sizes may be marketed. Each syringe is packaged in a sealed plastic tray. The tray also includes a needle for administering the injection.
Marketing Authorization Holder and Manufacturer
The marketing authorization holder is:
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Netherlands
Avonex is manufactured by:
FUJIFILM Diosynth Biotechnologies Denmark ApS
Biotek Allé 1,
DK-3400 Hillerød,
Denmark
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Netherlands
Contact your local representative if you want a version of this leaflet with larger text.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien Biogen Belgium NV/SA Tél: +32 2 2191218 | Lietuva Biogen Lithuania UAB Tel: +370 5 259 6176 |
| Luxembourg/Luxemburg Biogen Belgium NV/SA Tél: +32 2 2191218 |
Ceská republika Biogen (Czech Republic) s.r.o. Tel: +420 255 706 200 | Magyarország Biogen Hungary Kft. Tel: +36 1 899 9883 |
Danmark Biogen Denmark A/S Tlf.: +45 77 41 57 57 | Malta Pharma. MT Ltd. Tel: +356 21337008 |
Deutschland Biogen GmbH Tel: +49 (0) 89 99 6170 | Nederland Biogen Netherlands B.V. Tel: +31 20 542 2000 |
Eesti Biogen Estonia OÜ Tel: +372 618 9551 | Norge Biogen Norway AS Tlf: +47 23 40 01 00 |
Ελλάδα Genesis Pharma SA Τηλ: +30 210 8771500 | Österreich Biogen Austria GmbH Tel: +43 1 484 46 13 |
España Biogen Spain S.L. Tel: +34 91 310 7110 | Polska Biogen Poland Sp. z o.o. Tel: +48 22 351 51 00 |
France Biogen France SAS Tél: +33 (0)1 41 37 9595 | Portugal Biogen Portugal Sociedade Farmacêutica, Unipessoal Lda. Tel: +351 21 318 8450 |
Hrvatska Biogen Pharma d.o.o. Tel: +385 1 775 73 22 | România Johnson & Johnson Romania S.R.L. Tel: +40 21 207 18 00 |
Ireland Biogen Idec (Ireland) Ltd. Tel: +353 (0)1 463 7799 | Slovenija Biogen Pharma d.o.o. Tel: +386 1 511 02 90 |
Ísland Icepharma hf Sími: +354 540 8000 | Slovenská republika Biogen Slovakia s.r.o. Tel: +421 2 323 34008 |
Italia Biogen Italia s.r.l. Tel: +39 02 584 9901 | Suomi/Finland Biogen Finland Oy Puh/Tel: +358 207 401 200 |
Κύπρος Genesis Pharma Cyprus Ltd Τηλ: +357 22 76 57 15 | Sverige Biogen Sweden AB Tel: +46 8 594 113 60 |
Latvija Biogen Latvia SIA Tel: +371 68 688 158 |
Date of Last Revision of this Leaflet:08/2024.
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
This leaflet can be found in all languages of the European Union/European Economic Area on the European Medicines Agency website.
- How to Inject AVONEX
You should receive training on how to inject Avonex
These notes are a reminder. If you have any doubts, consult your doctor or pharmacist.
Where to Inject
- Avonex is injected into a muscle, for example, into the muscles of the upper thigh. It is not recommended to inject Avonex into the buttocks.
- Inject at a different site each week. This involves less risk of skin or muscle irritation.
- Do not useareas of skin with bruises, ulcers, or infections, or if there is an open wound.
Contents of the Plastic Tray
|
- Preparation
- Remove a sealed plastic tray from the refrigerator
- Check the expiration date on the tray lid. Do not use if it is expired.
- Completely remove the paper lid. Check that the blister tray contains a pre-filled syringe and an injection needle (see the image "Contents of the Plastic Tray").
- Allow the syringe to warm up
- Let it sit at room temperature for half an hour. This makes the injection more comfortable than if it were injected directly from the refrigerator.
Tip:do not use external heat sources, such as hot water, to warm up the syringe.
- Wash your handswith water and soap and dry them.
- Prepare alcohol-soaked cotton balls and adhesive bandages(not provided) if you need them.
Locate a clean and stable surface to place the necessary materialsfor the injection. Place the tray on it.
| |
| Inspect the liquid in the syringe It should be clear and colorless.If the solution is cloudy, not transparent, or contains particles in suspension, do not use the pre-filled syringe. |
| Remove the syringe protector The syringe has a white tamper-evident protector. Make sure the protector is intact and has not been opened. Do not use that syringe if you think the protector has been opened. Hold the syringe so that the white protector is facing upwards. Bend the protector at a right angle until it breaks. Do not touch the connection port. Do not press the plunger. |
| Connect the needle Open the needle to expose the connection port. Do not remove the protector. Press the needle onto the syringe. Turn it clockwise until it locks Tip:make sure the injection needle is firmly attached to the syringe. There may be leaks otherwise. If you have been instructed to gradually increase the dose of Avonex, you may need to use an Avostartclip dose adjustment device that your doctor will provide. For more details, talk to your doctor. Now remove the plastic protector from the needle.Do not turn it. Tip:if you turn the needle protector to remove it, you may also accidentally disconnect the needle. |
| |
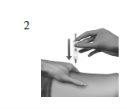
| Clean and stretch the injection site If necessary, use an alcohol-soaked cotton ball to clean the skin at the injection site you have chosen. Allow the skin to dry. With one hand, stretch the skin around the injection site. Relax your muscles. |
| Administer the injection Insert the injection needle into the muscle with a quick motion, like throwing a dart,at a right angle to the skin. The entire needle should be inserted. Slowly press the plunger until the syringe is empty. If you are using the syringe with an Avostartclip attached, you will receive a lower dose of Avonex. The syringe will not be completely empty. |
| Remove the needle Keep the skin perfectly stretched or compress the skin around the injection site and remove the needle. If you are using alcohol-soaked cotton balls, apply one to the injection site. If necessary, apply a bandage (adhesive bandage) over the injection site. Properly dispose of waste When you finish each injection, throw the needle and syringe into a special container (such as one for sharp objects), not into regular trash. If you have used the Avostartclip, the syringe (and Avostartclip) must be discarded after use. Do notuse the remaining Avonex. The used paper and cotton balls can be thrown into regular trash. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AVONEX 30 micrograms/0.5 ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 30 µgActive substance: interferon beta-1aManufacturer: Biogen Netherlands B.V.Prescription requiredDosage form: INJECTABLE, 22 µgActive substance: interferon beta-1aManufacturer: Merck Europe B.V.Prescription requiredDosage form: INJECTABLE, 44 µgActive substance: interferon beta-1aManufacturer: Merck Europe B.V.Prescription required
Online doctors for AVONEX 30 micrograms/0.5 ml INJECTABLE SOLUTION
Discuss questions about AVONEX 30 micrograms/0.5 ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions