
ATRACURIUM BESILATE KALCEKS 10 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION

How to use ATRACURIUM BESILATE KALCEKS 10 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Atracurium Besylate Kalceks 10 mg/ml Solution for Injection and Infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Atracurium Besylate Kalceks and what is it used for
- What you need to know before you are given Atracurium Besylate Kalceks
- How Atracurium Besylate Kalceks is used
- Possible side effects
- Storage of Atracurium Besylate Kalceks
- Contents of the pack and other information
1. What is Atracurium Besylate Kalceks and what is it used for
Atracurium Besylate belongs to a group of medicines called muscle relaxants.
Atracurium Besylate is used during surgical operations to relax muscles and to help with the insertion of a breathing tube and with artificial breathing. It is also used to assist with artificial breathing in patients admitted to the intensive care unit.
2. What you need to know before you are given Atracurium Besylate Kalceks
Atracurium Besylate Kalceks must not be given:
- if you are allergic to atracurium besylate or any of the other ingredients of this medicine (listed in section 6).
Tell your doctor before you are given atracurium besylate if this applies to you.
Warnings and precautions
Tell your doctor or nurse before you are given atracurium besylate:
- if you have an allergy or bronchial asthma;
- if you have ever had an allergic reaction to other medicines similar to atracurium besylate, which block the transmission of impulses between the nerve and the muscle;
- if you have muscle weakness, tiredness or difficulty coordinating your movements (myasthenia gravis);
- if you have a neuromuscular disease;
- if you have a heart disease or are sensitive to drops in blood pressure;
- if you have a significant electrolyte imbalance (unusual levels of ions such as sodium, potassium or chloride in the blood);
- if you have recently had severe burns that required medical attention.
If you think any of the above applies to you, talk to your doctor.
Children
This medicine is not intended for use in children under 1 month of age.
Other medicines and Atracurium Besylate Kalceks
Tell your doctor if you are taking, have recently taken or might take any other medicines, including those obtained without a prescription.
Some medicines may affect the effects of atracurium besylate. Tell your doctor if you are taking any of the following medicines:
- anaesthetics (used to reduce consciousness and pain during surgical procedures), such as halothane, isoflurane, enflurane or ketamine;
- antibiotics (used to treat infections), such as aminoglycosides, polymyxins, spectinomycin, tetracyclines, lincomycin and clindamycin;
- anti-arrhythmics (used to treat heart rhythm disorders), such as propranolol, oxprenolol, calcium channel blockers, lidocaine, procainamide and quinidine;
- diuretics, such as furosemide, mannitol, thiazides and acetazolamide;
- magnesium salts (used to prevent low magnesium levels in the body);
- medicines used to treat mental disorders, such as lithium or chlorpromazine;
- medicines used to treat high blood pressure (hypertension), such as trimethaphan and hexamethonium;
- medicines used to treat joint inflammation (anti-rheumatic drugs), such as chloroquine and penicillamine;
- medicines used to treat Alzheimer's disease, such as donepezil;
- corticosteroids (used to treat inflammation or asthma), such as prednisolone;
- medicines for seizures (epilepsy), such as phenytoin.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Your doctor will consider the benefit of your treatment with Atracurium Besylate Kalceks to your baby.
This medicine may be used to maintain muscle relaxation during a caesarean section.
Driving and using machines
Atracurium besylate may affect your ability to drive and use machines. Ask your doctor for advice on when it is safe to drive and use machines.
Do not drive or use machines if you do not feel well.
3. How Atracurium Besylate Kalceks is used
Atracurium besylate is used during procedures that require you to be anaesthetized (unconscious) or heavily sedated. It will always be given under the supervision of an experienced doctor.
How much is given
Your doctor will decide the correct dose of atracurium besylate based on:
- your body weight;
- the degree and duration of muscle relaxation required;
- your expected response (reaction) to the administration of the medicine.
How Atracurium Besylate Kalceks is given
This medicine will be given to you by a single injection into a vein or as a continuous infusion (usually using an infusion pump) into a vein. In this case, the medicine is given slowly over a certain period of time.
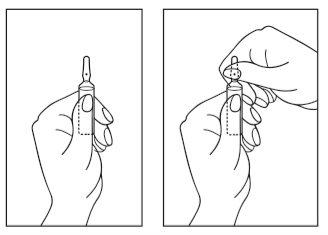
Instructions for opening the ampoule:
- Hold the ampoule with the colored dot facing upwards. If any solution remains in the top part of the ampoule, gently tap it with your finger to make the solution flow down to the bottom part of the ampoule.
- Use both hands to open it and while holding the bottom part of the ampoule with one hand, use the other hand to break the top part of the ampoule in the opposite direction of the colored dot (see the images below).
Use in children
Children under 1 month of age must not be given this medicine.
If you are given too much Atracurium Besylate Kalceks
This medicine should only be used by doctors who are properly trained in its administration. Since this medicine will be given to you while you are in the hospital, it is unlikely that you will be given too much or too little, however, tell your doctor or a healthcare professional immediately if you have any doubts. If you are given too much, the appropriate measures will be taken immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following happen, tell your doctor immediately:
- Severe allergic reaction – sudden rash with itching (urticaria), swelling of hands, feet, ankles, face, lips, mouth or throat (which may cause difficulty in swallowing or breathing), and you may feel like you are going to faint;
- shock;
- heart failure;
- cardiac arrest.
The above are very rare, serious side effects. You may need urgent medical attention.
Tell your doctor as soon as possible if you notice any of the following:
Common side effects(may affect up to 1 in 10 people):
- low blood pressure (hypotension), usually mild and temporary;
- redness of the skin.
Uncommon side effects(may affect up to 1 in 100 people):
- difficulty breathing and wheezing (bronchospasm).
Frequency not known(cannot be estimated from the available data):
- seizures;
- muscle disorders (myopathy), muscle weakness.
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Atracurium Besylate Kalceks
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton, after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Store in the original packaging to protect from light.
For single use only. Once opened, the product should be used immediately.
The solution should be inspected visually before use. It should only be used if the solution is clear and free of particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Atracurium Besylate Kalceks
- The active substance is atracurium besylate.
1 ml of solution contains 10 mg of atracurium besylate.
Each ampoule (2.5 ml) contains 25 mg of atracurium besylate.
Each ampoule (5 ml) contains 50 mg of atracurium besylate.
- The other ingredients are benzene sulfonic acid (for pH adjustment), water for injections.
Appearance of Atracurium Besylate Kalceks and pack contents
Clear, colorless or yellowish solution for injection and infusion, without visible particles.
Solution of 2.5 ml or 5.0 ml filled in type I glass ampoules of 5.0 ml.
The ampoules are placed in a PVC tray. The tray is packaged in a cardboard box.
Pack sizes: 1 or 5 ampoules.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
AS KALCEKS
Krustpils iela 71E,
Riga, LV‑1057,
Latvia
Tel.: +371 67083320
E‑mail: [email protected]
Manufacturer
Akciju sabiedriba “Kalceks”
Krustpils iela 71E,
Riga, LV‑1057,
Latvia
You can obtain further information on this medicine from the representative of the Marketing Authorisation Holder
EVER Pharma Therapeutics Spain SL
C/ Toledo 170
28005 Madrid
Spain
This medicine is authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Bulgaria Atracurium Kalceks 10 mg/ml ???????????/?????????? ???????
Belgium Atracurium Kalceks 10 mg/ml solution injectable/pour perfusion
Atracurium Kalceks 10 mg/ml oplossing voor injectie/infusie
Atracurium Kalceks 10 mg/ml Injektions-/Infusionslösung
Czech Republic Atracurium Kalceks
Estonia Atracurium besilate Kalceks
France ATRACURIUM KALCEKS 10 mg/ml, solution injectable/pour perfusion
Hungary Atracurium besilate Kalceks 10 mg/ml oldatos injekció/infúzió
Ireland Atracurium besilate 10 mg/ml solution for injection/infusion
Latvia Atracurium besilate Kalceks 10 mg/ml škidums injekcijam/infuzijam
Lithuania Atracurium besilate Kalceks 10 mg/ml injekcinis ar infuzinis tirpalas
Malta Atracurium Kalceks 10 mg/ml solution for injection/infusion
Netherlands Atracurium Kalceks 10 mg/ml oplossing voor injectie/infusie
Poland Atracurium Kalceks
Romania Atracurium Kalceks 10 mg/ml solu?ie injectabila/perfuzabila
Slovakia Atracurium Kalceks 10 mg/ml injekcný/infúzny roztok
Spain Atracurium Besylate Kalceks 10 mg/ml solution for injection and infusion EFG
Sweden Atracurium Kalceks 10 mg/ml injektions-/infusionsvätska, lösning
United Kingdom (Northern Ireland) Atracurium besilate 10 mg/ml solution for injection/infusion
Date of last revision of this leaflet: August 2022
---------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
For single use only. Once opened, the product should be used immediately.
From a microbiological point of view, unless the method of dilution prevents the risk of microbial contamination, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user.
Atracurium besylate is inactivated at high pH, so it should not be mixed in the same syringe with thiopental solutions or other alkaline solutions.
After injecting atracurium besylate into a small vein, it should be flushed through the vein with physiological solution. When other medicines are administered through the same needle or permanent cannula as atracurium besylate, it should be rinsed with a sufficient volume of physiological solution after administration of each medicine.
Atracurium Besylate Kalceks is a hypotonic solution and should not be administered in the same venous access as a blood transfusion.
Atracurium besylate is compatible with the following infusion solutions.
Infusion solution | Stability period |
Sodium chloride solution (9 mg/ml) for intravenous infusion | 24 hours |
Glucose solution (50 mg/ml) for intravenous infusion | 8 hours |
Ringer's solution for intravenous infusion | 8 hours |
Sodium chloride solution (1.8 mg/ml) and glucose solution (40 mg/ml) for intravenous infusion | 8 hours |
Ringer lactate solution for intravenous infusion | 4 hours |
When diluted in these solutions to obtain atracurium besylate concentrations of 0.5 mg/ml and above, the resulting solutions will be stable in daylight for the indicated periods at temperatures up to 25°C.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATRACURIUM BESILATE KALCEKS 10 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTIONDosage form: INJECTABLE, 10 mg atracurium besylateActive substance: atracuriumManufacturer: Hameln Pharma GmbhPrescription requiredDosage form: INJECTABLE, 10 mg atracurium besylate/mlActive substance: atracuriumManufacturer: Aspen Pharma Trading LimitedPrescription requiredDosage form: INJECTABLE, 10 mg/5 ml - 20 mg/10 mlActive substance: cisatracuriumManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for ATRACURIUM BESILATE KALCEKS 10 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION
Discuss questions about ATRACURIUM BESILATE KALCEKS 10 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















