
BERIATE 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION

How to use BERIATE 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Beriate 1000 IU powder and solvent for solution for injection and infusion
Human coagulation factor VIII
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet:
- What is Beriate and what is it used for
- What you need to know before you use Beriate
- How to use Beriate
- Possible side effects
- Storage of Beriate
- Contents of the pack and other information
1. What is Beriate and what is it used for
What is Beriate
Beriate is presented as a powder, accompanied by a solvent. The resulting solution should be administered into a vein, either by injection or by infusion.
Beriate is obtained from human plasma (which is the liquid part of the blood) and contains human coagulation factor VIII. It is used to prevent or stop bleeding caused by the lack of factor VIII (haemophilia A) in the blood. It can also be used to treat acquired factor VIII deficiency.
What Beriate is used for
Factor VIII is involved in blood coagulation. The lack of factor VIII means that the blood does not clot as quickly as it should, resulting in an increased tendency to bleed. Beriate provides factor VIII, which temporarily normalizes the coagulation mechanisms.
2. What you need to know before you use Beriate
The following paragraphs contain information that you and your doctor should consider before using Beriate.
Do not use Beriate:
- If you are allergic to human coagulation factor VIII or to any of the other components of this medicine (listed in section 6).
Warnings and precautions
Traceability
It is strongly recommended that each time you administer Beriate, you record the date of administration, the batch number, and the volume injected in your treatment diary.
Talk to your doctor or pharmacist before you start using Beriate
- It is possible that allergic-type hypersensitivity reactions may occur. Your doctor should inform you about the onset of early symptoms of hypersensitivity reactions, including hives, generalized urticaria, chest tightness, difficult breathing, low blood pressure, and anaphylaxis (a severe allergic reaction that causes serious breathing problems or dizziness). If you experience these symptoms, you should immediately stop administering the medicine and contact your doctor.
- The formation of inhibitors(antibodies) is a known complication that can occur during treatment with all medicines composed of factor VIII. These inhibitors, especially in large quantities, can prevent the treatment from working properly, so you and your child will be closely monitored for the development of such inhibitors. If your bleeding or your child's bleeding is not being controlled with Beriate, consult your doctor immediately.
- If you have a heart condition or are at risk of having one, inform your doctor or pharmacist.
- If a central venous access device (CVAD) is needed to administer Beriate, your doctor should consider the risk of complications related to the catheter, including local infections, bacterial infections (bacteraemia), and the formation of blood clots (thrombosis) at the catheter insertion site.
Your doctor will carefully weigh the benefits of treatment with Beriate against the risk of these complications.
Viral safety
When medicines derived from human blood or plasma are administered, certain measures are taken to prevent the transmission of infections to patients. This includes the careful selection of blood and plasma donors to exclude those who may pose a risk of transmitting infections, and the analysis of each donor and plasma pools for signs of viruses/infections. The manufacturers of these products also include stages in the production processes that can inactivate or eliminate viruses or other pathogens. Despite this, when medicines derived from human blood or plasma are administered, the possibility of transmitting infectious agents cannot be completely excluded. This also applies to emerging or unknown viruses and other pathogens.
These procedures are considered effective against enveloped viruses such as human immunodeficiency virus (HIV, AIDS virus), hepatitis B virus, and hepatitis C virus (liver inflammation), as well as against non-enveloped viruses such as hepatitis A virus and parvovirus B19.
Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly/repeatedly receive products derived from human plasma (e.g. factor VIII).
Using Beriate with other medicines:
- Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
- Beriate should not be mixed with other medicines, diluents, and solvents, except those recommended by the manufacturer (see section 6).
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- During pregnancy and breastfeeding, Beriate should only be used if clearly indicated.
- No information is available on fertility.
Driving and using machines
Beriate does not affect the ability to drive vehicles or use machines.
Beriate contains sodium
Beriate 1000 IU contains 27.55 mg of sodium (a major component of cooking/table salt) per vial. This is equivalent to 1.4% of the maximum daily intake of sodium recommended for an adult.
3. How to use Beriate
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are in doubt, consult your doctor or pharmacist again.
Treatment of haemophilia A should be started under the supervision of a doctor experienced in the treatment of this type of disorder.
Dosage
The dose of factor VIII you need and the duration of treatment depend on several factors, such as your body weight, the severity of your disease, the location and importance of the bleeding, or the need to prevent bleeding during surgery or a medical examination.
If you have been instructed to use Beriate at home, your doctor will ensure that you receive the necessary instructions on how to inject the product and how much product to use.
Follow the instructions given by your doctor or the nurses at your haemophilia centre.
Use in children and adolescents
The dose is calculated based on body weight and is determined in the same way as for adults.
If you use more Beriate than you should
No symptoms of overdose with factor VIII have been reported.
If you forget to use Beriate
Apply the next dose immediately and continue at regular intervals following your doctor's instructions. Do not take a double dose to make up for forgotten doses.
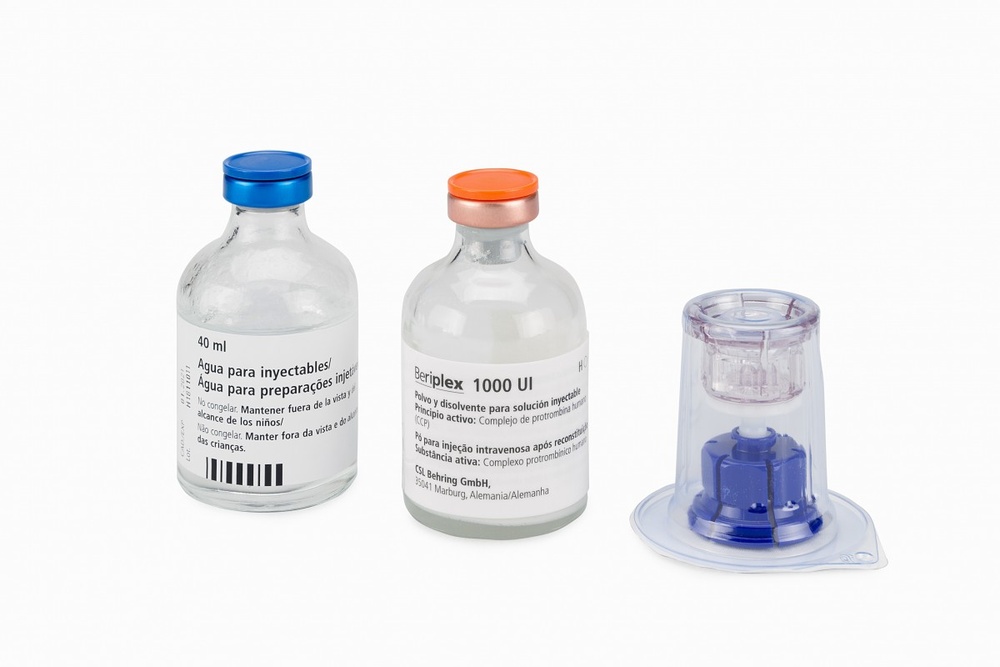
Reconstitution and administration
General instructions:
- The powder must be mixed (reconstituted) with the solvent (liquid) and withdrawn from the vial under aseptic conditions.
- The reconstituted solution should be clear or slightly opalescent, i.e. it may shine when held against a light. Occasionally, some flakes or particles may appear in the vial. The filter included in the Mix2Vial removes these particles. This filtration does not affect the dose calculations. After filtration and transfer of the reconstituted product to the syringe (see below), and before administration, the solution should be visually inspected to detect small particles and discolorations. Do not use solutions that are visibly turbid or contain flakes or particles in the syringe.
- Once the product is transferred to the syringe, it should be used immediately. Do notstore the product in the syringe.
- Unused product and waste materials should be disposed of properly according to local requirements and your doctor's instructions.
Reconstitution:
Allow the Beriate vials (vial with powder and vial with liquid) to reach room temperature without opening them. This can be done by leaving the vials at room temperature for 1 hour or by holding them in your hands for a few minutes. Do notexpose the vials to direct heat. The vials should not be heated above body temperature (37 °C).
Remove the protective caps from the vials containing the powder and solvent, and clean the exposed part of the stoppers with an alcohol swab. Allow the vials to dry before opening the Mix2Vial container, and follow the instructions below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Application and removal:
|
|
9 |
|
Use the venipuncture equipment provided with the product. Insert the needle into the vein. Allow blood to enter until the end of the tube. Connect the syringe to the threaded end of the venipuncture equipment. Slowly inject the reconstituted solution into the vein,following your doctor's instructions. The injection or infusion rate should not exceed 2 ml per minute. Be careful not to allow blood to enter the syringe containing the product.
If a large volume needs to be administered, infusion is an option to consider. The reconstituted product should be transferred to an authorized infusion system. Infusion should be performed according to your doctor's instructions.
Check yourself for any adverse effects that may occur immediately. If you experience any adverse effects related to the administration of Beriate, the injection or infusion should be interrupted (see also section 2).
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following side effects occur, consult your doctor immediately or go to the Emergency Department or the haemophilia centre at your nearest hospital:
- Symptoms of angioedema
- facial swelling, tongue or pharyngeal swelling,
- difficulty swallowing,
- hives and difficulty breathing.
These side effects have been observed very rarely (may affect up to 1 in 10,000 people), and in some cases may lead to severe allergic reactions (anaphylaxis), including shock
- Lack of effect (bleeding does not stop). In children who have not received previous treatment with factor VIII medicines, inhibitor antibodies (see section 2) may occur very frequently (more than 1 in 10 patients); however, in patients who have received previous treatment with factor VIII (more than 150 days of treatment), the risk is infrequent (less than 1 in 100 patients). If this happens, the medicines you or your child are taking may stop working properly, and you or your child may experience persistent bleeding. In this case, contact your doctor immediately.
Other side effects are:
- Allergic reactions (hypersensitivity), which may include:
- a burning sensation and itching at the injection or infusion site.
- chills, flushing, generalized skin rash, papules.
- headache.
- low blood pressure, unease, rapid heartbeat, chest tightness, difficult breathing.
- drowsiness (lethargy).
- nausea, vomiting.
- tingling.
These side effects have been observed very rarely, but in some cases may lead to severe allergic reactions (anaphylaxis), including shock.
- Fever has been observed very rarely.
Side effects in children and adolescents
The frequency, type, and severity of adverse reactions in children are expected to be the same as for adults.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Beriate
Do not use this medicine after the expiry date stated on the label and carton. The expiry date is the last day of the month stated.
- Store in a refrigerator (between 2 °C and 8 °C).
- During its validity period, Beriate can be stored at up to 25 °C for a maximum of 1 month. This storage period at room temperature should be recorded in your treatment diary to ensure the 1-month period is not exceeded.
- Beriate does not contain preservatives, so the reconstituted product should preferably be used immediately.
- If the reconstituted product is not administered immediately, storage in the vial at room temperature should not exceed 8 hours. Once transferred to the syringe, the product should be used immediately.
- Do not freeze.
- Keep the vial in the original packaging to protect it from light.
Keep out of the sight and reach of children.
6. Container Content and Additional Information
Composition of Beriate
The active ingredient is:
Beriate is presented as a powder (nominally containing 1000 IU of human coagulation factor VIII per vial) and a liquid (solvent). The reconstituted solution is administered either by injection or by perfusion.
Beriate 1000 IU is reconstituted with 10 ml of water for injectable preparations and contains approximately 100 IU/ml of human coagulation factor VIII.
The other components are:
Glycine, calcium chloride, sodium hydroxide (in small quantities) to adjust the pH, sucrose, and sodium chloride
Solvent: water for injectable preparations 10 ml.
Appearance of the Product and Container Content
Beriate is presented as a white powder and is supplied with the corresponding water for injectable preparations.
The reconstituted solution must be clear or slightly opalescent, i.e., it may shine when held against a light but must not contain particles.
Presentation:
Box with 1000 IU containing:
- 1 vial with powder
- 1 vial with 10 ml of Water for injectable preparations
- 1 transfer device with 20/20 filter
Administration equipment (inner box):
- 1 single-use 10 ml syringe
- 1 venipuncture device
- 2 alcohol-impregnated swabs
- 1 non-sterile dressing
Marketing Authorization Holder and Manufacturer
CSL Behring GmbH
Emil-von-Behring-Straße 76
35041 Marburg, Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
CSL Behring, S.A.
c/ Tarragona 157, 18th floor
08014 Barcelona
Spain
This medication is authorized in the member states of the European Economic Area with the following names:
Austria:
Beriate 100 I.E./ml Powder and solvent for solution for injection or infusion (250 I.E., 500 I.E., 1000 I.E.)
Beriate 200 I.E./ml Powder and solvent for solution for injection or infusion (2000 I.E.)
Bulgaria:
Beriate 250 IU Powder and solvent for solution for injection or infusion
Beriate 500 IU Powder and solvent for solution for injection or infusion
Beriate 1000 IU Powder and solvent for solution for injection or infusion
Beriate 2000 IU Powder and solvent for solution for injection or infusion
Croatia:
Beriate 250 IU powder and solvent for solution for injection or infusion
Beriate 500 IU powder and solvent for solution for injection or infusion
Beriate 1000 IU powder and solvent for solution for injection or infusion
Beriate 2000 IU powder and solvent for solution for injection or infusion
Czech Republic:
Beriate 250 IU, Beriate 500 IU, Beriate 1000 IU, Beriate 2000 IU
Estonia:
Beriate
Germany:
Beriate 250, Beriate 500, Beriate 1000, Beriate 2000
Hungary:
Presentations of Beriate 250, 500 and 1000:
BERIATE 100 NE/ml powder and solvent for solution for injection or infusion
Presentation 2000:
BERIATE 200 NE/ml powder and solvent for solution for injection or infusion
Italy:
Beriate
Latvia:
Beriate 250 SV powder and solvent for solution for injection or infusion
Beriate 500 SV powder and solvent for solution for injection or infusion
Beriate 1000 SV powder and solvent for solution for injection or infusion
Beriate 2000 SV powder and solvent for solution for injection or infusion
Lithuania:
Beriate® 250 TV powder and solvent for injectable or infusion solution
Beriate® 500 TV powder and solvent for injectable or infusion solution
Beriate® 1000 TV powder and solvent for injectable or infusion solution
Beriate® 2000 TV powder and solvent for injectable or infusion solution
Poland:
Beriate 250
Beriate 500
Beriate 1000
Beriate 2000
Portugal:
Beriate
Romania:
Beriate 250 powder and solvent for injectable/perfusion solution
Beriate 500 powder and solvent for injectable/perfusion solution
Beriate 1000 powder and solvent for injectable/perfusion solution
Beriate 2000 powder and solvent for injectable/perfusion solution
Spain:
Beriate 500 UI powder and solvent for injectable and perfusion solution
Beriate 1000 UI powder and solvent for injectable and perfusion solution
Beriate 2000 UI powder and solvent for injectable and perfusion solution
Slovakia:
Beriate 250 IU
Beriate 500 IU
Beriate 1000 IU
Beriate 2000 IU
Slovenia:
Beriate 250 i.e. powder and solvent for solution for injection/infusion
Beriate 500 i.e. powder and solvent for solution for injection/infusion
Beriate 1000 i.e. powder and solvent for solution for injection/infusion
Beriate 2000 i.e. powder and solvent for solution for injection/infusion
Date of the Last Revision of this Prospectus:April 2020
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)http://www.aemps.gob.es.
----------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals
Dosage
Treatment Monitoring
During treatment, it is recommended to properly monitor the levels of factor VIII to determine the dose to be administered and the frequency of repeated perfusions. The response of each patient to factor VIII may vary, demonstrating different half-lives and recoveries. The dose based on body weight may require an adjustment in patients with low weight or overweight. In the particular case of major surgical interventions, it is essential to monitor the substitution therapy accurately through coagulation tests (plasma activity of factor VIII).
Patients should be monitored for the development of factor VIII inhibitors. See also section 2.
The number of units of factor VIII administered is expressed in International Units (IU), in relation to the current standard of the World Health Organization (WHO) for factor VIII concentrates. The plasma activity of factor VIII is expressed as a percentage (in relation to normal human plasma) or preferably in IU (in relation to an international standard for factor VIII in plasma).
The activity of one IU of factor VIII is equivalent to the amount of factor VIII contained in one ml of normal human plasma.
On-demand Treatment
The calculation of the necessary dose of factor VIII is based on the empirical observation that 1 IU of factor VIII per kg of body weight increases the plasma activity of factor VIII by approximately 2% (2 IU/dL) over normal activity. The required dose is determined by the following formula:
Units required = body weight (kg) x desired increase in factor VIII [% or IU/dl] x 0.5
The amount administered and the frequency of administration will always be established based on the observed efficacy in each case.
In the following hemorrhagic episodes, the activity of factor VIII should not be less than the indicated plasma activity level (in % of normal level or IU/dL) during the corresponding period. The following table can be used as a dosage guide in hemorrhagic episodes and surgery.
Type of hemorrhagic episode/ Type of surgical procedure | Required factor VIII level (% or IU/dL) | Dosing frequency (hours) / Duration of therapy (days) |
Hemorrhage | ||
Early hemarthrosis, muscle bleeding, or oral cavity bleeding | 20-40 | Repeat every 12-24 hours. At least 1 day, until the hemorrhage is resolved, based on pain, or until adequate wound healing |
More extensive hemarthrosis, muscle bleeding, or hematoma | 30-60 | Repeat perfusion every 12-24 hours, for 3-4 days or more, until pain and acute disability are resolved |
Life-threatening hemorrhages | 60-100 | Repeat perfusion every 8-24 hours until the risk disappears |
Surgery | ||
Minor surgery, including dental extractions | 30-60 | Every 24 hours, at least 1 day, until wound healing |
Major Surgery | 80-100 (pre and postoperative) | Repeat perfusion every 8-24 hours until adequate wound healing; continue therapy for at least 7 more days to maintain a factor VIII activity of 30 to 60% (30-60 IU/dl corresponding to 0.30-0.60 IU/ml) |
Prophylaxis
For long-term prophylaxis of hemorrhages in patients with severe hemophilia A, the usual dose is 20 to 40 IU of factor VIII/kg of body weight at intervals of 2 to 3 days. In some cases, especially in young patients, it may be necessary to shorten the administration intervals or administer higher doses.
Pediatric Population
The dosage in pediatrics is based on body weight and therefore generally follows the same guidelines as for adults. The frequency of administration should always be oriented towards achieving clinical efficacy in each particular case. There is some experience in the treatment of children under 6 years of age.
Information on the Pharmacological Properties of the von Willebrand Factor
In addition to the protective function of factor VIII, the von Willebrand factor facilitates the adhesion of platelets at sites with vascular injury and plays a role in platelet aggregation.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BERIATE 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for BERIATE 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION
Discuss questions about BERIATE 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION AND FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





 1
1 2
2 3
3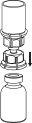 4
4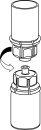 5
5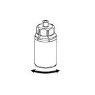 6
6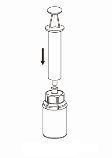 7
7 8
8









