
BANDOL 12.5 mg ORAL SUSPENSION

How to use BANDOL 12.5 mg ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Bandol12.5 mg/pulse, oral suspension
sildenafil
Read the entire package leaflet carefully before starting to take the medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Bandol and what is it used for
- What you need to know before taking Bandol
- How to take Bandol
- Possible side effects
- Storage of Bandol
- Package contents and additional information
1. What is Bandol and what is it used for
Bandol contains the active ingredient sildenafil, which belongs to a group of medications called phosphodiesterase type 5 (PDE5) inhibitors. It works by dilating the blood vessels of the penis, allowing blood flow when sexually stimulated. Bandol will only help you achieve an erection if you are sexually stimulated.
Bandol is indicated for the treatment of erectile dysfunction in adult men, sometimes called impotence. This occurs when a man cannot achieve or maintain a firm erection suitable for satisfactory sexual activity.
2. What you need to know before taking Bandol
Do not takeBandol
- If you are allergic to sildenafil or any of the other components of this medication (listed in section 6).
- If you are taking medications called nitrates, as the combination can lead to a dangerous decrease in your blood pressure. Consult your doctor if you are taking any of these medications, which are often administered to relieve angina pectoris (or "chest pain"). If you are unsure, consult your doctor or pharmacist.
- If you are using any medications called nitric oxide donors, such as amyl nitrite ("poppers"), as the combination can lead to a dangerous decrease in your blood pressure.
- If you are taking riociguat. This medication is used to treat pulmonary arterial hypertension (i.e., high blood pressure in the lungs) and chronic thromboembolic pulmonary hypertension (i.e., high blood pressure in the lungs caused by blood clots). PDE5 inhibitors, such as Bandol, have shown that they increase the hypotensive effect of this medication. If you are taking riociguat or are unsure, consult your doctor.
- If you have a serious heart or liver problem.
- If you have recently experienced a stroke or heart attack, or if you have low blood pressure.
- If you suffer from a rare inherited eye disease (such as retinitis pigmentosa).
- If you have previously experienced vision loss due to non-arteritic anterior ischemic optic neuropathy (NAION).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take Bandol:
- If you have sickle cell anemia (a red blood cell abnormality), leukemia (blood cell cancer), or multiple myeloma (bone marrow cancer).
- If you have a penile deformity or Peyronie's disease.
- If you have heart problems. Your doctor should carefully check if your heart can withstand the extra effort of having sex.
- If you currently have a stomach ulcer or bleeding problems (such as hemophilia).
- If you experience a sudden decrease or loss of vision, stop taking Bandol and contact your doctor immediately.
It is not recommended to use Bandol simultaneously with any other oral or local treatment for erectile dysfunction.
Do not take Bandol with treatments for pulmonary arterial hypertension (PAH) that contain sildenafil or any other PDE5 inhibitor.
Do not take Bandol if you do not have erectile dysfunction.
The use of Bandol is not indicated in women.
Special considerations in patients with kidney or liver problems
You should inform your doctor if you have kidney or liver problems. Your doctor may decide to reduce your dose.
Children and adolescents
The use of Bandol is not indicated in persons under 18 years of age.
Use ofBandolwith other medications
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Bandol may interfere with some medications, especially those used to treat chest pain. In case of a medical emergency, you should inform your doctor, pharmacist, or nurse that you are taking Bandol and when you took it. Do not take Bandol with other medications unless your doctor advises you to do so.
Do not take Bandol if you are taking medications called nitrates, as the combination of these medications can lead to a dangerous decrease in your blood pressure. Always inform your doctor, pharmacist, or nurse if you are taking any of these medications, which are often used to relieve angina pectoris (or "chest pain").
Do not take Bandol if you are taking medications called nitric oxide donors, such as amyl nitrite ("poppers"), as the combination of these medications can also lead to a dangerous decrease in your blood pressure.
Inform your doctor or pharmacist if you are taking riociguat.
If you are taking medications known as protease inhibitors, such as those used in the treatment of HIV, your doctor may recommend that you start treatment with the lowest dose (25 mg = 2 sprays) of Bandol.
Some patients who are receiving an alpha-blocker, a medication used to treat high blood pressure or prostate enlargement, may experience dizziness or lightheadedness that can be caused by a decrease in blood pressure when sitting or standing up quickly. Some patients have experienced these symptoms when taking Bandol with alpha-blockers. This is more likely to occur within 4 hours of taking Bandol.
In order to reduce the likelihood of these symptoms occurring, you should be receiving your daily dose of the alpha-blocker regularly before starting Bandol. Your doctor may indicate that you start treatment with the lower dose (1 ml equivalent to 25 mg of sildenafil, 2 sprays) of Bandol.
Inform your doctor or pharmacist if you are taking medications that contain sacubitril/valsartan, used to treat heart failure.
Use ofBandolwith food, drinks, and alcohol
Bandol can be taken with or without food. However, you may notice that Bandol takes a little longer to work if you take it with a heavy meal.
Consuming alcohol can temporarily make it more difficult to achieve an erection. Therefore, to get the maximum benefit from the medication, it is recommended not to consume large amounts of alcohol before taking Bandol.
Pregnancy, breastfeeding, and fertility
The use of Bandol is not indicated in women.
Driving and using machines
Bandol can cause dizziness and affect vision. You should know how you react to Bandol before driving vehicles or using machinery.
Bandol contains sodium benzoate (E-211)
This medication contains 1 mg of sodium benzoate (E-211) per milliliter of suspension.
3. How to take Bandol
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again. The recommended starting dose is 2 ml equivalent to 50 mg (4 sprays).
Each spray releases 0.5 ml of suspension containing 12.5 mg of sildenafil.
Number of sprays | Amount of suspension released | Amount of sildenafil |
2 | 1 ml | 25 mg |
4 | 2 ml | 50 mg |
8 | 4 ml | 100 mg |
The maximum daily dose is 4 ml equivalent to 100 mg of sildenafil (8 sprays).
Do not takeBandolmore than once a day.
Do not take Bandol with other medications that contain sildenafil.
You should take Bandol approximately one hour before you plan to have sex.
Shake vigorously before taking. The medication will be administered orally.
Before the first administration, it is necessary to perform 3 sprays, discarding the contents to prime the dosing pump. A lower dose may be administered without priming the dosing pump when used for the first time.
In no case will the formulation be administered via the nasal route.
Method of administration
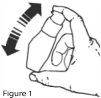
- The bottle should be shaken vigorously for the necessary time until no drug precipitate is observed in the bottle before each use. See Figure 1.
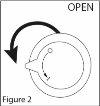
- Turn the dosing pump to the open position. See Figure 2.
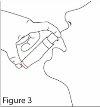
- Only for the first administration: give 3 sprays to prime the dosing pump and discard the product of the third spray (in the first two sprays, there is no product due to the design of the dosing pump). 4. Tilt your head slightly backwards. Place the dosing pump in your mouth. Press the dosing pump the required number of times, according to the dose prescribed by your doctor, and apply the suspension over your tongue and immediately afterwards swallow the suspension with saliva. Avoid direct contact between the tip of the dosing pump and the oral cavity and tongue. See Figure 3.
- Turn the dosing pump to the closed position. See Figure 4.
If you notice that the effect of Bandol is too strong or too weak, inform your doctor or pharmacist.
Bandol will only help you achieve an erection if you are sexually stimulated. The time it takes for Bandol to work varies from person to person, usually between half an hour and one hour. The effect of Bandol may be delayed if you take it with a heavy meal.
In the event that Bandol does not help you achieve an erection or if the erection is not maintained for a sufficient amount of time to complete sexual intercourse, consult your doctor.
If you take moreBandolthan you should
You may experience an increase in side effects and their severity. Doses above 100 mg do not increase efficacy.
Do not exceed the recommended dose ofBandolprescribed by your doctor.
Contact your doctor if you have taken more Bandol than advised.
If you have any further questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them. The side effects reported in association with the use of Bandol are generally mild to moderate and short-lived.
If you experience any of the following serious side effects, stop takingBandoland seek immediate medical attention:
- Allergic reaction - this occurs with low frequency(may affect up to 1 in 100 people).
The symptoms include sudden whistling when breathing, difficulty breathing or dizziness, swelling of the eyelids, face, lips, or throat.
- Chest pain - this occurs with low frequency.
If they occur during or after sex:
- Sit in a semi-upright position and try to relax.
- Do not use nitratesto treat chest pain.
- Prolonged and sometimes painful erections - this occurs rarely(may affect up to 1 in 1,000 people).
If you have an erection that lasts more than 4 hours, you should contact your doctor immediately.
- Sudden decrease or loss of vision - this occurs rarely.
- Severe skin reactions - this occurs rarely.
The symptoms may include severe scaling and swelling of the skin, blistering of the mouth, genitals, and around the eyes, as well as fever.
- Seizures or fits - this occurs rarely.
Other side effects:
Very common(may affect more than 1 in 10 people): headache.
Common(may affect up to 1 in 10 people): nausea, facial flushing, hot flushes (including a sudden feeling of heat in the upper body), indigestion, abnormal color perception, blurred vision, visual impairment, nasal congestion, and dizziness.
Uncommon(may affect up to 1 in 100 people): vomiting, skin rash, eye irritation, eye discharge/red eyes, eye pain, flashing lights, visual clarity, sensitivity to light, watery eyes, palpitations, rapid heartbeat, high blood pressure, low blood pressure, muscle pain, feeling of drowsiness, reduced sense of touch, vertigo, ringing in the ears, dry mouth, nasal stuffiness or congestion, inflammation of the nasal mucosa (including symptoms such as runny nose, sneezing, and nasal congestion), pain in the upper abdomen, gastroesophageal reflux disease (including symptoms such as heartburn), blood in the urine, pain in the arms or legs, nosebleeds, feeling of warmth, and feeling of fatigue.
Rare(may affect up to 1 in 1,000 people): fainting, stroke, heart attack, irregular heartbeat, transient decrease in blood flow to some parts of the brain, feeling of compression in the throat, numbness of the mouth, bleeding in the back of the eye, double vision, decreased visual acuity, abnormal sensation in the eye, eye or eyelid swelling, small particles or spots in the vision, seeing halos around lights, dilation of the pupil, change in color of the white of the eye, penile bleeding, presence of blood in the semen, dry nose, nasal swelling, feeling of irritability, and sudden decrease or loss of hearing.
During post-marketing experience with sildenafil, rare cases of unstable angina (heart disease) and sudden death have been reported. It is noteworthy that most men who experienced these side effects, although not all of them, had pre-existing heart problems before taking this medication. It is not possible to determine whether these side effects were directly related to sildenafil.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for human use medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Bandol
Keep this medication out of the sight and reach of children.
No special storage conditions are required.
Do not use this medication after the expiration date that appears on the box and on the bottle after CAD. The expiration date is the last day of the month indicated.
Once opened, the contents should be used within a maximum period of 12 months.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need in the SIGRE collection point at the pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Packaging Content and Additional Information
Composition ofBandol
- The active ingredient of Bandol is sildenafil. Each ml of suspension contains 25 mg of sildenafil (35.1 as citrate).
- The other components are: sodium benzoate (E-211), anhydrous citric acid, sucralose (E-955), acesulfame potassium (E-950), hypromellose (15 cP), xanthan gum, peppermint flavor 501.500 TP0504 (which contains corn maltodextrin, flavoring agents (menthofuran 0.6%, 0.2% pulegone, estragole 0.09%) and modified corn starch E-1450 (7.9%)), masking flavor SC241160 (which contains natural flavoring substances, sucralose E-955 (94.5%), potato maltodextrin and monoammonium glycyrrhizinate (0.4%)), purified water.
Appearance of the Product and Packaging Content
Bandol is white in color, with a minty aroma and flavor.
The primary packaging material of Bandol consists of 30 ml high-density polyethylene (HDPE) bottles with a 22 mm wide mouth, equipped with a 0.5 ml polypropylene dosing pump per spray.
Marketing Authorization Holder and Manufacturer
Holder
Aspargo Labs Italia S.r.l.
Via Po, 102
00198 Rome (RM)
Italy
Manufacturer
Zinereo Pharma, S.L.U.
A Relva, s/n
36410 O Porriño - Pontevedra
Spain
Phone: +34 986 345 200
Or
Edefarm, S.L.
Polígono Industrial Enchilagar del Rullo, 117,
Villamarchante, 46191 Valencia
Spain
Phone: +34 962793717
Or
Farmalider, S.A.
c/ Aragoneses, 2
28108 - Alcobendas (Madrid)
Spain
Phone: +34 91 661 23 35
This leaflet was approved in:November 2023
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BANDOL 12.5 mg ORAL SUSPENSIONDosage form: TABLET, 25 mgActive substance: sildenafilManufacturer: Adamed Pharma S.A.Prescription requiredDosage form: CHEWABLE TABLET, 100 mgActive substance: sildenafilManufacturer: Farmalider S.A.Prescription requiredDosage form: CHEWABLE TABLET, 25 mgActive substance: sildenafilManufacturer: Farmalider S.A.Prescription required
Online doctors for BANDOL 12.5 mg ORAL SUSPENSION
Discuss questions about BANDOL 12.5 mg ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






