AVONEX 30 micrograms/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PEN

How to use AVONEX 30 micrograms/0.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
AVONEX 30 micrograms/0.5 ml solution for injection in pre-filled pen
(interferon beta-1a)
Pre-filled Pen
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Even if you have used Avonex before, some of the information may have changed.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only, and you should not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are listed in this leaflet. See section 4.
(Information Notes)
This leaflet is updated from time to time.
Check for updates to this leaflet each time you renew your prescription.
(Information Notes)
If you have any further questions, ask your doctor or pharmacist.
Contents of the Package Leaflet
- What is AVONEX and what is it used for
- What you need to know before you start using AVONEX
- How to use AVONEX PEN
- Possible side effects
- Storage of AVONEX PEN
- Contents of the pack and further information
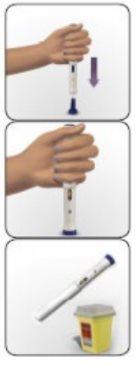
- How to inject yourself using AVONEX PEN
1. What is AVONEX and what is it used for
(Information Notes) Avonex works best when you use it:
Do not stop your treatment with Avonex without talking to your doctor first. |
What is AVONEX?
Avonex Pen is used to inject Avonex.The active substance of Avonex is a protein called interferon beta-1a.Interferons are natural substances produced by your body to protect you from infections and diseases. The Avonex protein is produced with exactly the same ingredients as the interferon beta present in the human body.
What is AVONEX used for?
Avonex is used to treat multiple sclerosis (MS).Treatment with Avonex may help prevent it from getting worse, although it will not cure MS.
Each patient has specific symptoms of MS.These may include:
- Feeling of instability or dizziness, walking problems, muscle stiffness and spasms, fatigue, tingling in the face, arms or legs
- Acute or chronic pain, bladder and bowel problems, sexual problems and vision problems
- Difficulty thinking and concentrating, depression.
MS also tends to flare up from time to time: this is what is known as a relapse.
Avonex may help reduce the number of relapses you may have and slow down the disabling effects of MS.Your doctor will explain how long you can use Avonex or when to stop treatment.
How does AVONEX work?
Multiple sclerosis is associated with nerve damage (in the brain or spinal cord). In MS, your body's defense system reacts against your own myelin, i.e., the insulation that surrounds nerve fibers. When myelin is damaged, messages between the brain and other parts of the body are disrupted. This is what causes the symptoms of MS. Avonex appears to work by preventing your body's defense system from attacking myelin.
2. What you need to know before you start using AVONEX
(Information Notes) Avonex and allergic reactions As Avonex is made from a protein, there is a small chance that an allergic reaction may occur. Additional information on depression Do not use Avonex if you have severe depression or have suicidal thoughts. If you have depression, your doctor may still prescribe Avonex, but it is important that you let them know if you have had depression or any other problem that affects your mood. |
Do not use AVONEX
- if you are allergicto interferon beta or any of the other ingredients of this medicine (listed in section 6);
- if you have severe depressionor have suicidal thoughts.
Talk to your doctor immediately in any of these cases.
Warnings and precautions
Talk to your doctor before you start using Avonex if you have or have had in the past:
- Depressionor problems that affect your mood
- Suicidal thoughts.
You must inform your doctor immediately of any changes in your mood, suicidal thoughts, unusual feelings of sadness, anxiety or hopelessness.
- Epilepsyor other uncontrolled seizure disorders
- Severe kidney or liver problems
- Low white blood cell or platelet counts,which may increase the risk of infection, bleeding or anemia
- Heart problemsthat may cause symptoms such as chest pain (angina),especially after any activity; swelling of ankles, difficulty breathing (congestive heart failure)or an irregular heartbeat (arrhythmias).
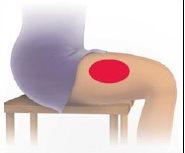
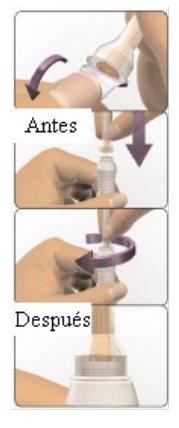
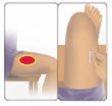
- Irritation at the injection site that can cause skin and tissue damage (injection site necrosis). When you are ready to administer the injection, carefully follow the instructions in section 7 "How to inject AVONEX PEN" at the end of this leaflet. This will reduce the risk of injection site reactions.
Talk to your doctor if you have any of these conditionsor if they get worse while you are using Avonex.
During treatment, blood clots may form in small blood vessels. These clots could affect your kidneys. This can happen after several weeks or several years after starting treatment with Avonex.
Your doctor may want to check your blood pressure, blood (platelet count) and kidney function.
Tell your doctor that you are using Avonex:
- If you are going to have a blood test.Avonex may interfere with the results.
(Information Notes) There may be times when you need to remind other healthcare professionals that you are being treated with Avonex.For example, if you are prescribed other medicines or if you have a blood test, Avonex may interact with other medicines or with the test results. |
Pediatric population
Avonex is not recommended for use in children and adolescents as the data on the use of Avonex in this population are limited. Avonex must not be used in children under 10 years of age because it has not been established whether it would work for them and whether it would be safe.
Other medicines and AVONEX
Tell your doctorif you are using, have recently used or might use any other medicines, especially for the treatment of epilepsy or depression. Avonex may affect other medicines or be affected by them. This also includes any medicine obtained without a prescription.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
No harmful effects on the baby are expected. Avonex can be used during breastfeeding.
Driving and using machines
If you feel dizzy, do not drive.Avonex can make some people feel dizzy. If this happens to you or if you experience any other side effect that may affect your ability, do not drive or use machines.
Important information about some of the ingredients of Avonex:
This medicine is essentially sodium-free. It contains less than 23 mg (1 mmol) of sodium per weekly dose.
3. How to use AVONEX PEN
(Information Notes) There is more information on how to inject yourself using Avonex Pen on the back of this leaflet. |
Recommended weekly dose
One injection using Avonex Pen, once a week.
Try to use Avonex at the same time on the same day each week.
Self-injection
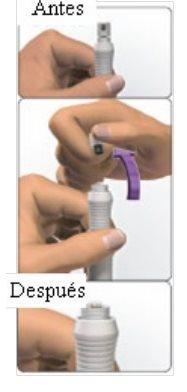
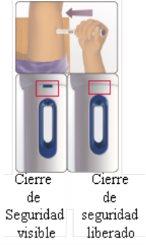
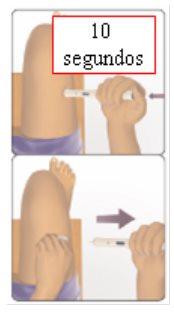
You can self-inject using Avonex Pen without the help of your doctor if you have been taught how to do it. See the self-injection instructions at the end of this leaflet (see section 7, How to inject yourself using AVONEX PEN).
If you have problemshandling Avonex Pen, ask your doctor for help.
Duration of treatment with AVONEX
Your doctor will tell you how long you should use Avonex. It is important that you use Avonex regularly. Do not make any changes that your doctor has not told you to make.
If you inject more AVONEX than you should
You should administer the injection using only one Avonex Pen, once a week. If you have used more than one Avonex Pen within three days, contact your doctor or pharmacist immediately for advice.
If you miss an injection
If you miss your usual weekly dose,inject a dose as soon as possible. Then wait a week before using Avonex Pen again. Continue with your injection on that day of each week. If you have a preferred day for using Avonex, talk to your doctor to adjust the dose and get back to your preferred day. Do not administer two injections to make up for the missed injection.
4. Possible Adverse Effects
(Informative Notes) Although the list of possible adverse effects may seem worrisome, it is possible that you will not experience any of them. |
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Severe adverse effects: seek medical help.
Severe allergic reactions
If you experience any of the following symptoms:
- Swelling of the face, lips, or tongue
- Difficulty breathing
- Rash.
Call a doctor immediately.Do not use Avonex until you have spoken with a doctor.
Depression
If you experience any symptoms of depression:
- Unusual feeling of sadness, anxiety, or hopelessness.
Call a doctor immediately.
Liver problems
If you experience any of the following symptoms:
- Yellowing of the skin or the white part of the eyes (jaundice)
- Generalized itching
- Feeling of discomfort (nausea and vomiting)
- Bruises that appear easily on the skin.
Call a doctor immediatelyas they may be manifestations of a possible liver problem.
Adverse effects observed in clinical trials.
(Informative Notes) Adverse effects observed in clinical trials. These are adverse effects reported by people during the evaluation of Avonex. The figures are based on the number of people who presented them. They give an idea of the likelihood that you will develop similar adverse effects. |
Very common adverse effects(may affect more than 1 in 10 people)
- Pseudoflu-like symptoms: headache, muscle pain, chills, or fever: see Pseudoflu-like symptoms, below
- Headache.
Common adverse effects(may affect up to 1 in 10 people)
- Lack of appetite
- Feeling of weakness and fatigue
- Difficulty sleeping
- Depression
- Facial flushing
- Nasal discharge
- Diarrhea (soft stools)
- Feeling of discomfort (nausea or vomiting)
- Numbness or tingling of the skin
- Rash, skin bruising
- Increased sweating, night sweats
- Pain in muscles, joints, arms, legs, or neck
- Muscle cramps, stiffness in muscles and joints
- Pain, bruising, and redness at the injection site
- Changes in blood tests. The symptoms you may experience are fatigue, repeated infections, bruising, or bleeding without apparent cause.
Uncommon adverse effects(may affect up to 1 in 100 people)
- Hair loss
- Changes in menstruation
- Burning sensation at the injection site.
Rare adverse effects(may affect up to 1 in 1,000 people)
- Difficulty breathing
- Kidney problems, including scarring that can reduce kidney function
If you experience any of the following symptoms:
- Foamy urine
- Fatigue
- Swelling, especially of ankles and eyelids, and weight gain.
Inform your doctor as they may be manifestations of a possible kidney problem.
- Blood clots in small blood vessels that can affect your kidneys (thrombotic thrombocytopenic purpura or hemolytic uremic syndrome). The symptoms may include an increase in bruising, bleeding, fever, extreme weakness, headache, dizziness, or fainting. Your doctor may find changes in your blood and kidney function.
If you are concerned about any of the effects, talk to your doctor.
Other adverse effects
(Informative Notes) These effects have been observed in people using Avonex, but we do not know how frequently they occur. If you feel dizzy, do not drive. |
- Thyroid dysfunction or excess activity
- Nervousness or anxiety, emotional instability, irrational thoughts or hallucinations (seeing or hearing things that are not real), confusion, or suicide
- Numbness, dizziness, seizures, or epileptic fits, and migraines
- Perception of your heartbeat (palpitations), rapid or irregular heartbeat, or heart problems accompanied by the following symptoms: reduced exercise capacity, inability to stay lying down, difficulty breathing, or swelling of ankles
- Liver problems, such as those described previously
- Urticaria or pseudovesicular rash, itching, worsening of existing psoriasis
- Swelling or bleeding at the injection site, tissue destruction (necrosis), or chest pain after an injection
- Weight gain or loss
- Changes in test results, such as variations in liver function tests
- Pulmonary arterial hypertension: a disease in which there is a significant narrowing of the blood vessels in the lungs, causing an increase in pressure in the blood vessels that carry blood from the heart to the lungs. Pulmonary arterial hypertension was observed at different times, even several years after starting treatment with interferon beta-containing medications.
If you are concerned about any of the effects, talk to your doctor.
Injection site effects
- Fainting sensation:The first injection of Avonex may be administered by your doctor. You may feel dizzy. You may even faint. It is unlikely to happen again.
- Immediately after the injection, you may notice tense or very weak muscles,as if you were going to repeat the experience. It is rare. It only occurs when self-injecting, and the effects pass quickly. They can occur at any time after starting treatment with Avonex.
- If you notice any skin irritation or other skin problemafter an injection, talk to your doctor.
Pseudoflu-like symptoms
(Informative Notes) Three simple ways to reduce the impact of pseudoflu-like symptoms:
|
Some people report that after using Avonex Pen, they feel like they have the flu.
The signs are:
- Headache
- Muscle pain
- Chills or fever.
These symptoms do not actually correspond to the flu
You cannot transmit them to anyone. They are more frequent when using Avonex for the first time. The pseudoflu-like symptoms gradually decrease as more injections are administered.
Children (10 years of age or older) and adolescents
In clinical trials, some adverse effects were reported more frequently in children and adolescents than in adults, e.g., muscle pain, pain in a limb, fatigue, and joint pain.
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
In order to improve the traceability of this medicine, your doctor or pharmacist must record the name and batch number of the medicine administered to you in your medical history. You may also want to take note of this information in case you are asked for it in the future.
5. Storage of Avonex Pen
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date shown on the label.
Avonex Pen contains a pre-filled syringe of Avonex. Store in the original packaging to
protect it from light.
Store in the refrigerator (between 2°C and 8°C). Do not freeze.
Avonex Pen can also be stored at room temperature (between 15°C and 30°C) for a
maximum of one week.
Do not use Avonex Pen if you observe that:
- The pen is broken.
- The solution has color and you can see particles floating.
- The closure guarantee capsule is broken.
Medicines should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Contents and Additional Information
Composition of AVONEX PEN
The active ingredientis: interferon beta-1a 30 micrograms/0.5 ml.
The other componentsare: sodium acetate trihydrate, glacial acetic acid, arginine hydrochloride, polysorbate 20, and water for injectables.
Appearance of AVONEX PEN and Container Contents
Each individual container contains an Avonex Pen, a needle, and a pen protector. Avonex Pen contains a pre-loaded Avonex syringe and should only be used after receiving proper training. Avonex Pen is available in packs of four or twelve, for one month or three months of injection.
Marketing Authorization Holder and Manufacturer
The marketing authorization holder is:
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Netherlands
Avonex is manufactured by:
FUJIFILM Diosynth Biotechnologies Denmark ApS
Biotek Allé 1,
DK-3400 Hillerød,
Denmark
Biogen Netherlands B.V.
Prins Mauritslaan 13
1171 LP Badhoevedorp
Netherlands
Contact your local representative if you want a version of this leaflet with larger text.
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
België/Belgique/Belgien Biogen Belgium NV/SA Tel: +32 2 2191218 | Lietuva Biogen Lithuania UAB Tel: +370 5 259 6176 |
| Luxembourg/Luxemburg Biogen Belgium NV/SA Tel: +32 2 2191218 |
Ceská republika Biogen (Czech Republic) s.r.o. Tel: +420 255 706 200 | Magyarország Biogen Hungary Kft. Tel.: +36 1 899 9883 |
Danmark Biogen Denmark A/S Tlf.: +45 77 41 57 57 | Malta Pharma. MT Ltd. Tel: +356 21337008 |
Deutschland Biogen GmbH Tel: +49 (0) 89 99 6170 | Nederland Biogen Netherlands B.V. Tel: +31 20 542 2000 |
Eesti Biogen Estonia OÜ Tel: +372 618 9551 | Norge Biogen Norway AS Tlf: +47 23 40 01 00 |
Ελλάδα Genesis Pharma SA Τηλ: +30 210 8771500 | Österreich Biogen Austria GmbH Tel: +43 1 484 46 13 |
España Biogen Spain S.L. Tel: +34 91 310 7110 | Polska Biogen Poland Sp. z o.o. Tel: +48 22 351 51 00 |
France Biogen France SAS Tél: +33 (0)1 41 37 9595 | Portugal Biogen Portugal Sociedade Farmacêutica, Unipessoal Lda. Tel: +351 21 318 8450 |
Hrvatska Biogen Pharma d.o.o. Tel: +385 1 775 73 22 | România Johnson & Johnson Romania S.R.L. Tel: +40 21 207 18 00 |
Ireland Biogen Idec (Ireland) Ltd. Tel: +353 (0)1 463 7799 | Slovenija Biogen Pharma d.o.o. Tel: +386 1 511 02 90 |
Ísland Icepharma hf Sími: +354 540 8000 | Slovenská republika Biogen Slovakia s.r.o. Tel: +421 2 323 34008 |
Italia Biogen Italia s.r.l. Tel: +39 02 584 9901 | Suomi/Finland Biogen Finland Oy Puh/Tel: +358 207 401 200 |
Κύπρος Genesis Pharma Cyprus Ltd Τηλ: +357 22 76 57 15 | Sverige Biogen Sweden AB Tel: +46 8 594 113 60 |
Latvija Biogen Latvia SIA Tel: +371 68 688 158 |
Date of last revision of this leaflet:08/2024.
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
This leaflet can be found in all languages of the European Union/European Economic Area on the European Medicines Agency website.
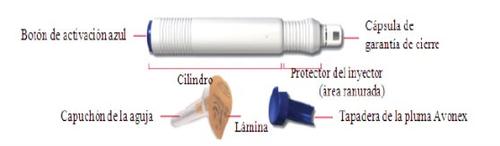
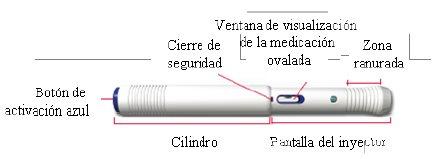
- How to inject using AVONEX PEN
Avonex Pen (single use)
Container contents – Avonex Pen, needle, and Avonex Pen protector
|