
ARANESP 100 micrograms INJECTABLE SOLUTION IN PRE-FILLED PEN

How to use ARANESP 100 micrograms INJECTABLE SOLUTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Aranesp 10 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 15 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 20 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 30 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 40 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 50 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 60 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 80 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 100 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 130 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 150 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 300 micrograms solution for injection in pre-filled pen (SureClick)
Aranesp 500 micrograms solution for injection in pre-filled pen (SureClick)
darbepoetin alfa (darbepoetin alfa)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Aranesp and what is it used for
- What you need to know before you use Aranesp
- How to use Aranesp
- Possible side effects
- Storage of Aranesp
- Contents of the pack and other information
1. What is Aranesp and what is it used for
Your doctor has prescribed Aranesp (an anti-anemic) for the treatment of your anemia. Anemia occurs when the blood does not contain enough red blood cells and the symptoms can be fatigue, weakness, and shortness of breath.
Aranesp works exactly like the natural hormone erythropoietin. Erythropoietin is produced in the kidneys and helps the bone marrow to produce more red blood cells. The active substance of Aranesp is darbepoetin alfa, produced by genetic technology in Chinese Hamster Ovary (CHO-K1) cells.
If you have chronic kidney disease
Aranesp is used to treat symptomatic anemia associated with chronic kidney disease (kidney failure) in adults and children. In kidney failure, the kidney does not produce enough of the natural hormone erythropoietin, which can often cause anemia.
As your body will need some time to produce more red blood cells, it will take about four weeks before you notice any effect. Your normal dialysis routine will not affect Aranesp's ability to treat anemia.
If you are receiving chemotherapy
Aranesp is used to treat symptomatic anemia in adult patients with non-myeloid tumors treated with chemotherapy.
One of the main adverse reactions of chemotherapy is that it makes the bone marrow stop producing enough red blood cells. Towards the end of chemotherapy treatment, especially if you have received a lot of chemotherapy, your red blood cell count may decrease, causing anemia.
2. What you need to know before you use Aranesp
Do not use Aranesp:
- if you are allergic to darbepoetin alfa or any of the other ingredients of this medicine (listed in section 6).
- if you have high blood pressure that is not being treated with other medicines prescribed by your doctor.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Aranesp.
Tell your doctor if you have or have had:
- high blood pressure that is being treated with medicines prescribed by your doctor;
- sickle cell anemia;
- seizures (fits);
- convulsions (spasms and fits);
- liver disease;
- if you do not respond to medicines used to treat anemia;
- allergy to latex (the needle cap of the pre-filled pen contains a latex derivative); or
- hepatitis C.
Special precautions:
- If you experience symptoms such as more tiredness than usual and lack of energy, it may be due to a disorder called pure red cell aplasia (PRCA) that has been observed in some patients. PRCA means that the body has reduced or stopped the production of red blood cells, causing severe anemia. If you experience these symptoms, contact your doctor, who will determine the best way to treat your anemia.
- Be especially careful with other medicines that stimulate the production of red blood cells: Aranesp belongs to a group of medicines that stimulate the production of red blood cells, such as human erythropoietic proteins. Your doctor should always keep a record of the exact medicine you are using.
- If you are a patient with chronic kidney disease and especially if you do not respond correctly to Aranesp, your doctor will review the dose of Aranesp, as if you do not respond to treatment, repeated increases in the dose of Aranesp could increase the risk of having a heart or blood vessel problem and could increase the risk of heart attack, stroke, and death.
- Your doctor will try to keep your hemoglobin levels between 10 and 12 g/dl. Your doctor will check that your hemoglobin does not exceed a certain level, as high concentrations of hemoglobin could put you at risk of having a heart or blood vessel problem and could increase the risk of heart attack, stroke, and death.
- If you experience symptoms that include severe headache, numbness, confusion, vision problems, nausea, vomiting, or seizures (convulsions), it may mean that you have very high blood pressure. If you experience these symptoms, you should contact your doctor.
- If you are a cancer patient, you should know that Aranesp may act as a growth factor for blood cells and that in some circumstances it may have a negative effect on your cancer. Depending on your individual situation, it may be preferable to have a blood transfusion. Please discuss this with your doctor.
- The use of this medicine in healthy subjects can cause heart or blood vessel problems that can be fatal.
- Severe skin reactions have been reported in association with epoetin treatment, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). SJS/TEN can appear initially as red spots similar to a target or circular spots often with central blisters on the torso. Additionally, ulcers can occur in the mouth, throat, nose, genitals, and eyes (red and swollen eyes). These severe rashes are often preceded by fever or flu-like symptoms. The rashes can progress to widespread skin peeling and potentially fatal complications.
If you experience a severe rash or any of these skin symptoms, stop taking Aranesp and inform your doctor or seek immediate medical attention.
Using Aranesp with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Ciclosporin and tacrolimus (medicines that suppress the immune system) may be affected by the number of red blood cells in the blood. It is important that you tell your doctor if you are using any of these medicines.
Using Aranesp with food and drinks
Food and drink do not affect Aranesp.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Aranesp has not been used in pregnant women. It is important that you tell your doctor if you:
- are pregnant;
- think you may be pregnant; or
- are planning to have a baby.
It is not known whether darbepoetin alfa is excreted in breast milk. If you are treated with Aranesp, you should stop breastfeeding.
Driving and using machines
Aranesp should not affect your ability to drive or use machines.
Aranesp contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, i.e., it is essentially "sodium-free".
3. How to use Aranesp
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
After some blood tests, your doctor has decided that you need Aranesp because your hemoglobin level is equal to or less than 10 g/dl. Your injection should be administered under the skin (subcutaneously), so you should use Aranesp pre-filled pen. Your doctor will tell you how much Aranesp you need and how often you should use it to keep your hemoglobin level between 10 and 12 g/dl. This may vary depending on whether you are an adult or a child.
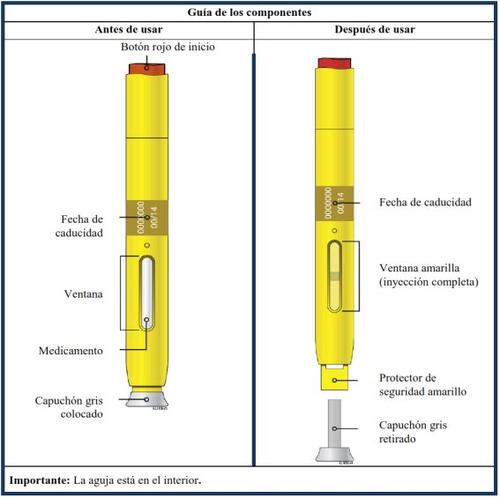
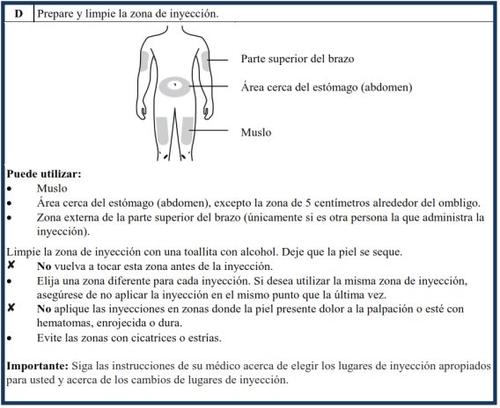
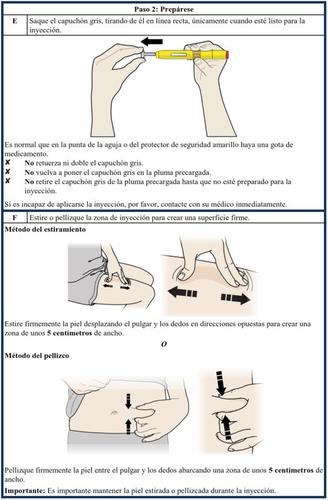
How to inject Aranesp yourself
Your doctor has decided that the Aranesp pre-filled pen is the best way for you, your nurse, or another person to administer Aranesp to you. Your doctor, nurse, or pharmacist will teach you how to inject yourself with the pre-filled syringe. Do not try to inject yourself if you have not been taught how to do it. Never inject Aranesp yourself into a vein. The pre-filled pen is designed to be injected only under the skin.
For information on how to use the pre-filled pen, read the instructions at the end of this leaflet.
If you have chronic kidney disease
For all adult and pediatric patients ≥ 1 year of age with chronic kidney disease, Aranesp pre-filled pen is administered in a single injection under the skin (subcutaneously).
To correct anemia, the initial dose of Aranesp per kilogram of body weight will be:
- 0.75 micrograms once every two weeks, or
- 0.45 micrograms once a week.
For adult patients not on dialysis, 1.5 micrograms/kg once a month may also be used as the initial dose.
For all adult and pediatric patients ≥ 1 year of age with chronic kidney disease, once anemia is corrected, you will continue to receive Aranesp in a single injection, either once a week or once every 2 weeks. For all adult and pediatric patients ≥ 11 years of age who are not on dialysis, Aranesp may also be administered as a monthly injection.
Your doctor will regularly take blood samples to see how anemia is responding to treatment and, if necessary, may need to adjust the dose every four weeks to maintain long-term control of your anemia.
Your doctor will use the lowest effective dose to control the symptoms of your anemia.
If you do not respond adequately to Aranesp, your doctor will review your dose and inform you if you need to change the doses of Aranesp.
Your doctor will regularly check your blood pressure, especially at the start of treatment.
In some cases, your doctor may recommend that you take iron supplements.
Your doctor may decide to change the way you are given the injection (under the skin or in a vein). If this happens, you will start with the same dose you were receiving before and will have blood tests to check that anemia is still being treated correctly.
If your doctor has decided to change your treatment from r-HuEPO (genetically engineered erythropoietin) to Aranesp, they will choose to administer Aranesp either once a week or once every two weeks. The route of administration will be the same as with r-HuEPO, but your doctor will decide how much and when you should be given it, and may adjust the dose you receive if necessary.
If you are receiving chemotherapy
Aranesp is administered under the skin in a single injection, once a week or once every three weeks.
To correct anemia, the initial dose of Aranesp will be:
- 500 micrograms once every three weeks (6.75 micrograms of Aranesp per kilogram of body weight), or
- 2.25 micrograms of Aranesp per kilogram of body weight (once a week).
Your doctor will regularly take blood samples to measure how anemia is responding, and may adjust the dose as necessary. Treatment will continue until approximately 4 weeks after the end of chemotherapy. Your doctor will tell you exactly when to stop taking Aranesp.
In some cases, your doctor may recommend that you take iron supplements.
If you use more Aranesp than you should
You may have serious problems if you administer more Aranesp than you should, such as very high blood pressure. Contact your doctor or pharmacist if this happens. If you do not feel well, contact your doctor or pharmacist immediately.
If you forget to use Aranesp
Do not take a double dose to make up for forgotten doses.
If you forget to inject a dose of Aranesp, contact your doctor to see when you should inject the next dose.
If you stop using Aranesp
If you want to stop using Aranesp, you should first discuss it with your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some patients have experienced the following side effects using Aranesp:
Patients with chronic kidney disease
Very common:may affect more than 1 in 10 people
- High blood pressure (hypertension)
- Allergic reactions
Common:may affect up to 1 in 10 people
- Stroke
- Pain at the injection site
- Rash and/or redness of the skin
Uncommon:may affect up to 1 in 100 people
- Blood clots (thrombosis)
- Seizures (fits)
Frequency not known:frequency cannot be estimated from the available data
- Pure red cell aplasia (PRCA) – (anemia, more tiredness than usual, lack of energy)
Patients with cancer
Very common:may affect more than 1 in 10 people
- Allergic reactions
- Fluid retention (edema)
Common:may affect up to 1 in 10 people
- High blood pressure (hypertension)
- Blood clots (thrombosis)
- Pain at the injection site
- Rash and/or redness of the skin
Uncommon:may affect up to 1 in 100 people
- Seizures (fits)
All patients
Frequency not known:frequency cannot be estimated from the available data
- Severe allergic reactions that can include:
- Unexpected allergic reactions that can be life-threatening (anaphylaxis)
- Swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing or breathing (angioedema)
- Difficulty breathing (allergic bronchospasm)
- Skin rashes
- Hives (urticaria)
- Severe skin reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in association with epoetin treatment. These can appear as red spots similar to a target or circular spots often with central blisters on the torso, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes, and can be preceded by fever or flu-like symptoms.
Stop taking Aranesp if you experience these symptoms and inform your doctor or seek immediate medical attention. See also section 2.
- Bruising and bleeding at the injection site
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Aranesp
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the pre-filled pen after “EXP”. The expiry date refers to the last day of the month shown.
Store in a refrigerator (2°C - 8°C). Do not freeze. Do not use Aranesp if you think it has been frozen.
Keep the pre-filled pen in the outer packaging to protect it from light.
Once you have removed the pre-filled pen from the refrigerator and left it at room temperature for about 30 minutes before injecting, you must use it within 7 days or discard it.
Do not use this medicine if you notice that the contents of the pre-filled pen are cloudy or contain particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Aranesp Composition
- The active ingredient is darbepoetina alfa, r-HuEPO (erythropoietin produced by genetic technology). The pre-filled syringes contain 10, 15, 20, 30, 40, 50, 60, 80, 100, 130, 150, 300 or 500 micrograms of darbepoetina alfa.
- The other components are sodium phosphate monobasic, sodium phosphate dibasic, sodium chloride, polysorbate 80, and water for injectable preparations.
Product Appearance and Package Contents
Aranesp is a clear, colorless or slightly opalescent injectable solution in a pre-filled syringe.
Aranesp (SureClick) is available in packs of 1 or 4 pre-filled syringes. Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Amgen Europe B.V.
Minervum 7061
NL-4817 ZK Breda
Netherlands
Marketing Authorization Holder
Amgen Europe B.V.
Minervum 7061
NL-4817 ZK Breda
Netherlands
Manufacturer
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co Dublin
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
For further information about this medicinal product, please contact the local representative of the marketing authorization holder.
België/Belgique/Belgien s.a. Amgen n.v. Tel/Tél: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 |
???????? ?????? ???????? ???? ???.: +359 (0)2 424 7440 | Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tel/Tél: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen, filial af Amgen AB, Sverige Tlf: +45 39617500 | Malta Amgen B.V. The Netherlands Tel: +31 (0)76 5732500 |
Deutschland AMGEN GmbH Tel.: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tel: +47 23308000 |
Ελλ?δα Amgen Ελλ?ς Φαρμακευτικ? Ε.Π.Ε. Τηλ.: +30 210 3447000 | Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220550 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Limited United Kingdom Tel: +44 (0)1223 420305 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Puh/Tel: +358 (0)9 54900500 |
K?προς C.A. Papaellinas Ltd Τηλ.: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 | United Kingdom Amgen Limited Tel: +44 (0)1223 420305 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu.
This leaflet is available in all EU/EEA languages on the European Medicines Agency website.
Instructions for Use
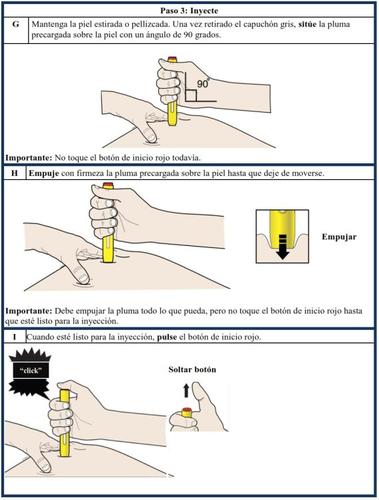
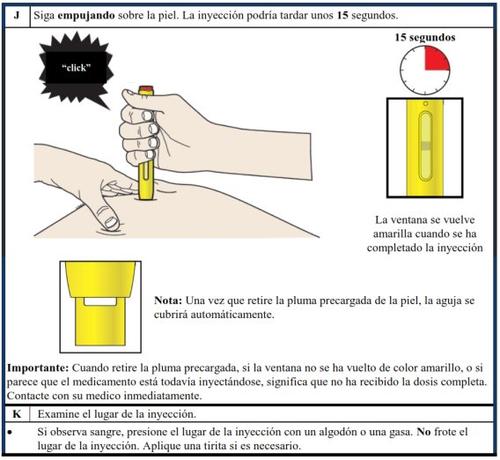
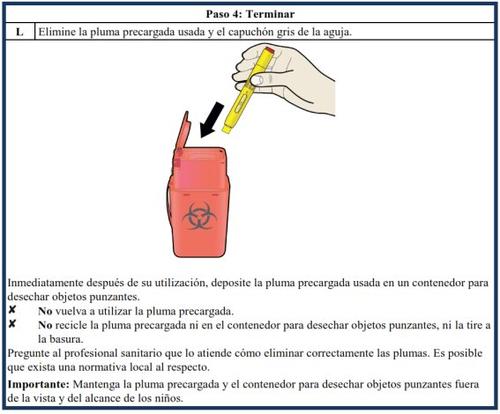
It is important that you do not attempt to administer the injection yourself or have a caregiver administer it to you if you have not received training from a healthcare professional.
Additional educational materials are available to provide training on how to self-administer the Aranesp pre-filled syringe, a demonstration device, and a patient/caregiver instruction poster for those with visual impairments.


- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ARANESP 100 micrograms INJECTABLE SOLUTION IN PRE-FILLED PENDosage form: INJECTABLE, UnknownActive substance: darbepoetin alfaManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: darbepoetin alfaManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 100 µgActive substance: darbepoetin alfaManufacturer: Amgen Europe B.V.Prescription required
Online doctors for ARANESP 100 micrograms INJECTABLE SOLUTION IN PRE-FILLED PEN
Discuss questions about ARANESP 100 micrograms INJECTABLE SOLUTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















