ANAPEN 0.30 mg/0.3 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use ANAPEN 0.30 mg/0.3 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Anapen 0.30 mg/0.3 ml Solution for Injection in Pre-filled Syringe
Adrenaline (Epinephrine)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Pack
- What Anapen is and what it is used for
- What you need to know before you use Anapen
- How to use Anapen
- Possible side effects
- Storing Anapen
- Contents of the pack and other information

1. What Anapen is and what it is used for
- Anapen is presented as a pre-filled syringe of adrenaline in an automatic injection system (Autoinjector). This system injects a single dose of adrenaline into the muscle.
- This medicine is for emergency situations only and you should see your doctor immediately after using your Autoinjector.
after using your Autoinjector.
- Adrenaline is a hormone that is naturally released in response to stress. In acute allergic reactions, it works by improving blood pressure, heart function, and breathing, and reducing swelling. Adrenaline is also known as epinephrine.
- Anapen is used for the emergency treatment of severe allergic reactions (anaphylaxis) caused by peanuts or other foods, medicines, insect bites or stings, and other allergens, as well as exercise or an unknown cause.
2. What you need to know before you use Anapen
Do not use Anapen
There is no known reason why Anapen should not be used in an emergency allergic situation.
Warnings and Precautions
Talk to your doctor or pharmacist before you start using Anapen.
- Your doctor should instruct you thoroughly on when and how to use the Anapen Autoinjector correctly.
- You should tell your doctor if you have a heart condition, including angina, hyperthyroidism, high blood pressure, low potassium levels, and increased calcium in the blood, circulatory problems, pheochromocytoma (a type of tumor in the adrenal gland), increased eye pressure (glaucoma), kidney or prostate disease, diabetes, or any other disease.
- If you have asthma, you may have an increased risk of a severe allergic reaction.
- Anyone who has had an anaphylactic episode should see their doctor to determine what substances they may be allergic to, in order to strictly avoid them in the future. It is also important to note that an allergy to one substance can lead to allergies to a large number of related substances.
- If you have food allergies, it is essential to review the ingredients of everything you are going to ingest (including medicines), as even small amounts can cause severe reactions.
- Repeated local injection can cause skin damage at the injection site. Accidental intravascular injection can cause a sudden increase in blood pressure. Accidental injection into hands or feet can cause loss of blood flow to the affected area. You should go to the nearest emergency room immediately.
Using Anapen with other medicines
Tell your doctor or pharmacist if you are using or have recently used or may need to use any other medicines, including:
- Medicines for heart disease, such as digitalis (digoxin), beta-blockers, quinidine,
- Medicines for depression, such as tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), serotonin and noradrenaline reuptake inhibitors (SNRIs),
- Medicines for diabetes, your doctor may adjust the dose of your medication after using Anapen,
- Medicines for Parkinson's disease,
- Medicines for thyroid disorders,
- Other medicines: antihistamines such as diphenhydramine or chlorpheniramine, theophylline, ipratropium and oxitropium (used to treat respiratory diseases such as asthma), oxytocin (used in pregnant women's contractions), inhaled anesthetics, alpha-adrenergic blockers (used to treat high blood pressure), sympathomimetics (used in the treatment of asthma, other respiratory diseases, and nasal congestion).
Using Anapen with alcohol
Alcohol may negatively affect this medicine by increasing its effects.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
- It is not clear if the administration of adrenaline during pregnancy poses a risk to the fetus. This should not stop you from using Anapen in an emergency, as your life may be at risk. Consult your doctor before an emergency occurs.
- Adrenaline is not expected to have any effect on the breastfed infant.
Driving and using machines
You should not drive or operate machinery after injecting this medicine, as you may still be experiencing the effects of anaphylactic shock.
Anapen contains sodium metabisulfite (E223)
This medicine may cause severe allergic reactions and bronchospasm (sudden feeling of suffocation) because it contains sodium metabisulfite (E223).
You should tell your doctor or pharmacist if you know you are allergic to sodium metabisulfite.
Anapen contains a small amount of sodium chloride (salt)
This medicine contains less than 23 mg (1 mmol) of sodium per dose, so it is considered essentially "sodium-free".
3. How to use Anapen
Always carry two auto-injectors with you in case the first administration fails or a dose is not sufficient.
- Follow your doctor's instructions for administering this medicine exactly.
- If you are unsure, consult your doctor or pharmacist again.
- It is injected only into the thigh.
- For single use, please ensure that you dispose of it safely immediately after use. Anapen releases a single dose of 0.3 ml of liquid that is equivalent to 0.3 mg (300 micrograms) of adrenaline. After use, 0.75 ml of volume remains in the auto-injector, which should not be reused.
The reaction usually starts minutes after exposure to the allergen, and the person may experience:
- Skin itching, hives, redness, and swelling of the eyes, lips, or tongue.
- Difficulty breathing due to throat swelling. Wheezing, shortness of breath, and coughing due to hardening of the lung muscles.
- Other symptoms of anaphylaxis, such as headaches, vomiting, and diarrhea.
- Collapse and loss of consciousness due to a sudden drop in blood pressure.
If you experience these signs and symptoms, use the Anapen Autoinjector immediately. You should only inject this medicine into the outer part of the thigh, never into the buttock.
Sometimes, a single dose of adrenaline may not be enough to completely reverse the effects of a severe allergic reaction. For this reason, your doctor may prescribe more than one unit of Anapen. If your symptoms have not improved or have worsened 5-15 minutes after the first injection, either you or the person you are with should administer a second injection. For this reason, you should always carry more than one unit of Anapen with you.
Use in adults
- The recommended dose is 0.30 mg for people weighing less than 60 kg.
- The recommended dose is 0.30 mg to 0.50 mg for people weighing more than 60 kg, according to clinical criteria.
- Adults weighing more than 60 kg may need more than one injection to reverse the effect of an allergic reaction, according to clinical criteria.
Use in children and adolescents
- The appropriate dose is 0.15 mg or 0.30 mg,
- This depends on the child's body weight and the doctor's criteria.
- Children and adolescents weighing more than 30 kg should use Anapen 0.30 mg.
- Auto-injectors that release 0.15 mg of adrenaline are also available. A dose below 0.15 mg cannot be administered with sufficient accuracy in children weighing less than 15 kg, so its use is not recommended unless the situation poses a risk to their health and under medical criteria.
Children between 15 kg and 30 kg
The usual dose is 0.15 mg.
Children over 30 kg
The usual dose is 0.30 mg.
Anapen is designed as an emergency treatment. You should always seek medical help immediatelyafter using Anapen. Call 112, request an ambulance, and indicate "anaphylaxis", even if symptoms seem to be improving. You will need to go to the hospital for observation and further treatment, as necessary. This is because the reaction can recur later.
While waiting for the ambulance, you should lie down with your feet raised, unless this makes breathing difficult, in which case you should remain seated. Ask someone to stay with you until the ambulance arrives, in case you become unwell again.
Unconscious patients should be placed in the recovery position.
Instructions for use
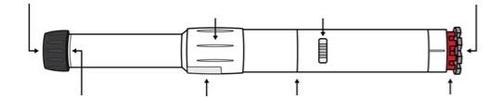
- Parts of the Anapen Autoinjector
Before using the Anapen Autoinjector, you need to know all the parts of the Autoinjector. These are shown in the drawing.
Black needle protector (reversible) | Rotating solution window cover | Injection indicator | Gray safety cap |
| |||
Autoinjector needle end | Solution window | Safety lock | Red injector button |
- Rotating solution window cover: Turn the cover over the solution window to align the lens with the solution window of the Autoinjector.
- Solution window: Look through the lens of this window before injection to check that the solution is clear and colorless.
- Injection indicator: Before injection, you can see a white plastic plunger through the window. This indicates that the Anapen Autoinjector has not been activated by mistake or tampered with. After injection, the injection indicator turns red, indicating that the Autoinjector has been activated correctly.
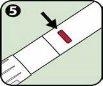
- Black needle protector (reversible): Protects the needle when you are not using the Anapen Autoinjector. Pull the needle protector off before injection. After injection, the patient should turn the black needle protector back and put it back on the same end of the Anapen Autoinjector to cover the needle.
- Gray safety cap: Covers the red injector button. Prevents the button from being pressed by mistake.
Do not remove the black needle protector or the gray safety cap until the Anapen Autoinjector is ready to be used.
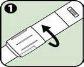
- Checking the Anapen Autoinjector
Before using your Anapen Autoinjector, you should perform the following checks:
|
|
|
Discard the Anapen Autoinjector if the solution is cloudy, colored, or contains particles. |
|
|
|
|
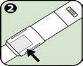
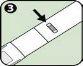
- Using the Anapen Autoinjector
If you have removed the black needle protector, do not place your thumb, fingers, or hand over the open end (needle end) of the Anapen Autoinjector.
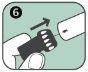
Follow these steps to use the Anapen Autoinjector:
|
|
|
|
|
|
|
|
|
If it is not red, you should repeat the injection with a new Anapen. |
|
|
Return your used Anapen to the hospital or pharmacy for proper disposal.
If you use more Anapen than you should
- If you inject too much adrenaline or do so accidentally into a blood vessel or finger, you should seek immediate medical help at the nearest hospital.
- In case of overdose or accidental injection, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20.
- If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
- Anapen contains sodium metabisulfite (E223) which may cause allergic reactions and difficulty breathing, especially in those with a history of asthma. You should seek medical help immediately if you experience these side effects.
- Common side effects of adrenaline include a feeling of strong heartbeat (palpitations), fast or irregular heartbeat, sweating, nausea, vomiting, difficulty breathing, dizziness, weakness, paleness, tremors, headaches, apprehension, nervousness, anxiety, and coldness in the extremities.
- Less common side effects include: hallucinations, fainting, dilated pupils, difficulty urinating, muscle tremors, increased blood pressure, and blood changes such as high sugar levels, low potassium levels, and high acid content.
- Occasionally, at high doses, or in susceptible patients, a sudden increase in blood pressure may occur, which can cause cerebral hemorrhage, irregular heartbeat, or heart attack, and decreased blood flow to the skin, mucous membranes, and kidneys.
Reporting side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Anapen
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and the auto-injector. The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C.
Do not use this medicine if you notice that the solution is cloudy, changes color, or contains particles. See the instructions for use to know how to check the medicine.
Keep the auto-injector in the outer packaging to protect it from light.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Anapen
- The active ingredient is adrenaline (epinephrine) 0.30 mg in 0.3 ml.
- The other components are: sodium metabisulfite (E223), sodium chloride, hydrochloric acid, water for injectable preparations.
Appearance of the product and package contents
Anapen is presented in the form of a pre-filled syringe with an adrenaline solution for injection in an automatic injection system (Auto-injector).
Packages with 1 or 2 auto-injector units with a thermoformed protective tray in the cardboard box are available. Only some package sizes may be marketed.
Length of the exposed needle: 10 mm ± 1.5 mm.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Bioprojet Pharma,
9 rue Rameau 75002 Paris, France
Manufacturer:
Owen Mumford Limited
Primsdown Industrial Estate,
Worcester Road, Chipping Norton,
Oxfordshire OX7 5XP,
UK
LYOFAL - SALON DE PROVENCE, ZA La Gandonne,
452 rue du Rémoulaire, SALON DE PROVENCE,
13300, France
This medicine is authorized in the member states of the European Economic Area under the following names:
Austria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Portugal, Romania, Slovenia, Spain, Sweden, and the Netherlands: Anapen
Belgium, Italy: Chenpen
Date of the last revision of this prospectus:04/2024
Anapen is a registered trademark.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price42.09 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ANAPEN 0.30 mg/0.3 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 1 mg/10 mlActive substance: epinephrineManufacturer: Laboratoire AguettantPrescription requiredDosage form: INJECTABLE, Epinephrine base 1 mg/mlActive substance: epinephrineManufacturer: B Braun Medical S.A.Prescription requiredDosage form: INJECTABLE, 1 mg/mlActive substance: epinephrineManufacturer: Laboratorios Basi Industria Farmaceutica S.A.Prescription required
Online doctors for ANAPEN 0.30 mg/0.3 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about ANAPEN 0.30 mg/0.3 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions