
ALZERTA 13.3 mg/24h TRANSDERMAL PATCHES

How to use ALZERTA 13.3 mg/24h TRANSDERMAL PATCHES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Alzerta 13.3 mg/24 h Transdermal Patches EFG
Rivastigmine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Alzerta and what is it used for
- What you need to know before you use Alzerta
- How to use Alzerta
- Possible side effects
- Storing Alzerta
- Contents of the pack and other information
1. What is Alzerta and what is it used for
The active substance of Alzerta is rivastigmine.
Rivastigmine belongs to a group of medicines called cholinesterase inhibitors. In patients with Alzheimer's dementia, certain nerve cells die in the brain, causing low levels of the neurotransmitter acetylcholine (a substance that allows nerve cells to communicate with each other). Rivastigmine works by blocking the enzymes that break down acetylcholine: acetylcholinesterase and butyrylcholinesterase. By blocking these enzymes, rivastigmine allows the increase of acetylcholine in the brain, helping to reduce the symptoms of Alzheimer's disease.
Alzerta is used for the treatment of adult patients with mild to moderately severe Alzheimer's dementia, a progressive brain disorder that gradually affects memory, intellectual ability, and behavior.
2. What you need to know before you use Alzerta
Do not use Alzerta
- if you are allergic to rivastigmine (the active substance of Alzerta) or any of the other ingredients of this medicine (listed in section 6).
- if you have ever had an allergic reaction to a similar medicine (carbamate derivatives).
- if you have a skin reaction that spreads beyond the patch size, if there is a more intense local reaction (such as blisters, skin inflammation, swelling) and if there is no improvement within 48 hours after removing the transdermal patch.
If you are in any of these situations, inform your doctor and do not use Alzerta transdermal patches.
Warnings and precautions
Consult your doctor before starting to use Alzerta:
- if you have or have ever had any heart problems, such as irregular or slow heart rate, QTc prolongation, family history of QTc prolongation, torsades de pointes, or if you have low levels of potassium or magnesium in your blood.
- if you have or have ever had an active stomach ulcer.
- if you have or have ever had difficulties urinating.
- if you have or have ever had seizures.
- if you have or have ever had asthma or severe respiratory disease.
- if you suffer from tremors.
- if you have low body weight.
- if you have gastrointestinal reactions such as nausea, vomiting, and diarrhea. You may become dehydrated (lose a large amount of fluid) if vomiting or diarrhea is prolonged.
- if you have liver problems (liver failure).
If you are in any of these situations, your doctor may consider it necessary to monitor you more closely while you are being treated.
If you have not used the patches for more than three days, do not put on another one without consulting your doctor first.
Children and adolescents
There is no experience with the use of this medicine in the pediatric population for the treatment of Alzheimer's disease.
Other medicines and Alzerta
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
This medicine may interfere with anticholinergic medicines, some of which are medicines used to relieve stomach cramps or spasms (e.g., dicyclomine), for the treatment of Parkinson's disease (e.g., amantadine), or to prevent motion sickness (e.g., diphenhydramine, scopolamine, or meclizine).
This medicine should not be administered at the same time as metoclopramide (a medicine used to relieve or prevent nausea and vomiting). Taking the two medicines together may cause problems such as stiffness in the limbs and hand tremors.
If you are going to have surgery while using this medicine, inform your doctor that you are using it, as it may potentiate the effects of some muscle relaxants used in anesthesia.
Caution should be exercised when using this medicine with beta-blockers (medicines such as atenolol used to treat hypertension, angina, and other heart conditions). Taking the two medicines together may cause complications such as a decrease in heart rate (bradycardia) that can lead to fainting or loss of consciousness.
Caution should be exercised when using Alzerta with other medicines that may affect heart rate or the heart's electrical system (QT prolongation).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
If you are pregnant, it is necessary to evaluate the benefits of using this medicine against the possible adverse effects for the fetus. This medicine should not be used during pregnancy unless clearly necessary.
You should not breastfeed while being treated with this medicine.
Driving and using machines
Your doctor will inform you if your illness allows you to drive or use machines safely. This medicine may cause dizziness and severe confusion. If you feel dizzy or confused, do not drive or use machines, or perform other tasks that require your attention.
3. How to use Alzerta
Follow exactly the instructions for administration of this medicine given by your doctor. If you are in doubt, consult your doctor or pharmacist again.
How to start treatment
Your doctor will tell you which dose of this medicine is most suitable for you.
- Normally, treatment with Alzerta starts with 4.6 mg/24 h.
- The recommended daily dose is Alzerta 9.5 mg/24 h. If this dose is well tolerated, the doctor treating you may consider increasing the dose to 13.3 mg/24 h.
- Only wear one patch of this medicine at a time and replace the patch with a new one every 24 hours.
During treatment, your doctor may adjust the dose depending on your individual needs.
If you have not used the patches for more than three days, do not put on another one without consulting your doctor first. Treatment with the transdermal patch can be restarted at the same dose if treatment is not interrupted for more than three days. Otherwise, your doctor will have you restart your treatment with Alzerta 4.6 mg/24 h.
This medicine can be used with food, drink, and alcohol.
Where to apply your Alzerta transdermal patch
- Before applying a patch, make sure the skin is clean, dry, and hairless, without powders, oils, moisturizers, or lotions that may prevent the patch from sticking well to the skin, without cuts, redness, or irritation.
- Remove carefully any patch you are already wearing before applying a new one.Wearing multiple patches on your body could expose you to an excessive amount of this medicine, which could be potentially dangerous.
- Apply only ONEpatch per day to only ONEof the possible areas as shown in the following diagrams:
- upper part of the left orright arm
- upper left orupper right part of the chest (avoiding the breasts in women)
- upper left orupper right part of the back
- lower left orlower right part of the back
Every 24hours, remove the previous patch before applying a new patch to only one of the following possible areas. |
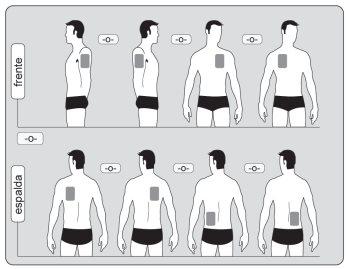
Each time you change the patch, remove the previous day's patch before applying a new patch to a different area of skin (e.g., one day on the right side of the body and the next day on the left side; or one day on the upper part of the body and the next day on the lower part). Wait at least 14 days before applying a new patch to exactly the same area of skin.
How to apply your Alzerta transdermal patch
Alzerta patches are thin, brown, and stick to the skin. Each patch is in a pouch that protects it until you are ready to apply it. Do not open the pouch or remove the patch until you are ready to apply it
| Remove carefully any existing patch before applying a new one. Patients who are starting treatment for the first time and patients who are restarting treatment with rivastigmine after an interruption of treatment should start with the second figure. |
| Each patch is in an individual protective pouch. Only open the pouch when you are ready to apply the patch. Cut the pouch with scissors along the dotted line, but not beyond the indicated line. Open the pouch. Do not cut the entire length of the pouch to avoid damaging the patch. Remove the patch from the pouch. |
| A protective film covers the adhesive side of the patch. Remove the first sheet of the protective film without touching the adhesive side of the patch with your fingers. |
| Place the adhesive side of the patch on the upper or lower back or on the upper arm or chest and then remove the second sheet of the protective film. |
| Press the patch firmly against the skin with the palm of your hand for at least 30 seconds and make sure the edges are well stuck. |
IMPORTANT:
- Remove the previous patch before applying a new one.
- Only one patch per day.
- Do not cut the patch into pieces.
- Press the patch firmly against the skin with the palm of your hand for at least 30seconds.
If it helps, you can write on the patch, for example, the day of the week, with a fine-tip pen.
You should wear the patch continuously until it is time to change it for a new one. When you apply a new patch, you can try different areas to find the ones that are most comfortable for you and where clothing does not rub against the patch.
How to remove your Alzerta transdermal patch
Gently pull one of the edges of the patch to slowly peel it off the skin. If there are any adhesive residues on the skin, soak the area with warm water and mild soap or use baby oil to remove it. Do not use alcohol or other solvents (nail polish removers or other solvents).
After removing the patch, wash your hands with soap and water. If you come into contact with your eyes or if your eyes become red after handling the patch, rinse them immediately with plenty of water and seek medical advice if the symptoms do not resolve.
Can you wear your Alzerta transdermal patch when bathing, swimming, or exposing yourself to the sun?
- Bathing, swimming, or showering should not affect the patch. Make sure it does not peel off partially while you perform these activities.
- Do not expose the patch to an external heat source (e.g., excessive sunlight, sauna, solarium) for long periods.
What to do if a patch falls off
If a patch falls off, apply a new one for the rest of that day and change it the next day at the usual time.
When and for how long should you wear your Alzerta transdermal patch?
- To benefit from your treatment, you should apply a new patch every day, preferably at the same time.
- Only wear one patch of this medicine at a time and replace the patch with a new one every 24 hours.
If you use more Alzerta than you should
If you accidentally apply more than one patch, remove all patches from the skin and inform your doctor or call the Toxicology Information Service, phone: 91 562 04 20 (indicating the medicine and the amount administered). You may need medical attention. Some people who have accidentally taken excessive amounts of rivastigmine have experienced nausea, vomiting, diarrhea, high blood pressure, and hallucinations. There may also be a slowing of the heart rate and fainting.
If you forget to use Alzerta
If you realize you have forgotten to apply a patch, apply it immediately. The next day, apply the next patch at the usual time. Do not apply two patches to make up for the one you forgot.
If you stop treatment with Alzerta
Tell your doctor or pharmacist if you stop using the patches.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, Alzerta transdermal patches may produce adverse effects, although not all people suffer from them.
You may have adverse effects more frequently when starting your treatment or when your dose is increased. Generally, adverse effects will slowly disappear as your body gets used to the medicine.
If you notice any of the following adverse effects that may be serious, remove the patch and immediately inform your doctor.
Frequent(may affect up to 1 in 10 people)
- Loss of appetite
- Feeling of dizziness
- Feeling of agitation or numbness
- Urinary incontinence (inability to properly stop urinating).
Infrequent(may affect up to 1 in 100 people)
- Heart rhythm problems such as slow heart rate
- Seeing things that really do not exist (hallucinations)
- Stomach ulcer
- Dehydration (loss of a large amount of fluid)
- Hyperactivity (high level of activity, restlessness)
- Aggressiveness
Rare(may affect up to 1 in 1,000 people)
- Falls
Very Rare(may affect up to 1 in 10,000 people)
- Stiffness of the arms and legs
- Tremor in the hands
Unknown(cannot be estimated from the available data)
- Allergic reaction where the patch was applied, such as blisters or skin inflammation
- Worsening of Parkinson's disease signs – such as tremor, stiffness, and difficulty moving
- Pisa syndrome (a condition that involves involuntary muscle contraction and abnormal tilting of the body and head to one side)
- Pancreatitis – signs include pain in the upper part of the stomach, often accompanied by a feeling of nausea or vomiting
- Fast or irregular heart rate
- High blood pressure
- Epileptic seizures (convulsions)
- Liver disorders (yellowing of the skin, yellowing of the whites of the eyes, abnormal darkening of urine or unexplained nausea, vomiting, fatigue, and loss of appetite)
- Changes in tests that show liver function
- Feeling of restlessness
- Nightmares
If you notice any of the adverse effects listed above, remove the patch and immediately inform your doctor.
Other adverse effects experienced with rivastigmine capsules or oral solution and that may occur with patches:
Frequent(may affect up to 1 in 10 people)
- Excessive saliva
- Loss of appetite
- Feeling of agitation
- Feeling of general discomfort
- Tremor or feeling of confusion
- Increased sweating
Infrequent(may affect up to 1 in 100 people)
- Irregular heart rate (e.g., fast heart rate)
- Difficulty sleeping
- Accidental falls
Rare(may affect up to 1 in 1,000 people)
- Epileptic seizures (convulsions)
- Ulcer in the intestine
- Chest pain – probably caused by heart spasm
Very Rare(may affect up to 1 in 10,000 people)
- High blood pressure
- Pancreatitis – signs include severe pain in the upper part of the stomach, often with a feeling of nausea or vomiting
- Gastrointestinal bleeding – manifested as blood in the stool or when vomiting
- Seeing things that do not exist (hallucinations)
- Some people who have suffered from intense vomiting have had a tear in part of the digestive tube that connects their mouth to their stomach (esophagus)
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines, Website: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Alzerta
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiration date that appears on the box and on the envelope after CAD. The expiration date is the last day of the month indicated.
- Keep the transdermal patch inside the envelope until use.
- Do not use any patch if you observe that it is damaged or shows signs of tampering.
After removing a patch, fold it in half with the adhesive side facing inward and press. After introducing it into the original envelope, dispose of the patch. Do not touch your eyes with your fingers and wash your hands with soap and water after removing the patch. If your household waste is eliminated by incineration, you can throw the patch in your household trash.
Medicines should not be thrown down the drain or into the trash. Deposit the containers and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Alzerta
The active ingredient is rivastigmine.
Each transdermal patch releases 13.3 mg of rivastigmine in 24 hours. Each 12.8 cm2 transdermal patch contains 19.2 mg of rivastigmine.
The other components are polyester film coated with polyethylene/thermoplastic resin/aluminum, poly[(2-ethylhexyl) acrylate, vinyl acetate], polyisobutene of medium and high molecular weight, anhydrous colloidal silica, light liquid paraffin, polyester film coated with fluoropolymer, orange printing ink.
Appearance of the Product and Package Contents
Each transdermal patch is a thin circular patch. The outer layer is brown and is printed in orange ink: “RIV-TDS 13.3 mg/24h”.
Each transdermal patch comes sealed in an envelope.
Alzerta 13.3 mg/24 h transdermal patches EFG are available in packages containing 7 or 30 envelopes and in multiple packages containing 60 (2x30) or 90 (3x30) envelopes.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
Spain
Manufacturer
Luye Pharma AG
Am Windfeld 35
83714 Miesbach
Germany
Date of the Last Revision of this Prospectus:December2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALZERTA 13.3 mg/24h TRANSDERMAL PATCHESDosage form: TRANSDERMAL PATCH, 4.6 mg/24 hActive substance: rivastigmineManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: TRANSDERMAL PATCH, 9.5 mg/24 hActive substance: rivastigmineManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: TRANSDERMAL PATCH, 4.6 mg/24 hActive substance: rivastigmineManufacturer: Esteve Pharmaceuticals S.A.Prescription required
Online doctors for ALZERTA 13.3 mg/24h TRANSDERMAL PATCHES
Discuss questions about ALZERTA 13.3 mg/24h TRANSDERMAL PATCHES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions


















