
ACULAR 5 mg/ml EYE DROPS SOLUTION

How to use ACULAR 5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
ACULAR 5 mg/ml Eye Drops Solution
(ketorolaco trometamol)
Read this entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist or nurse.
- This medication has been prescribed to you only and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet:
- What is ACULAR and what is it used for
- What you need to know before starting to use ACULAR
- How to use ACULAR
- Possible side effects
5 Conservation of ACULAR
- Package Contents and Additional Information
1. What is ACULAR and what is it used for
ACULAR is used to prevent and reduce ocular inflammation after cataract surgery in adults.
ACULAR belongs to a group of medications known as non-steroidal anti-inflammatory drugs (NSAIDs).
2. What you need to know before starting to use ACULAR
Do not use ACULAR
- If you are allergic to ketorolaco trometamol or any of the other ingredients of this medication (listed in section 6).
- If you are allergic to aspirin or other similar medications, such as other non-steroidal anti-inflammatory drugs.
Warnings and Precautions
Consult your doctor, pharmacist, or nurse before starting to use ACULAR.
If you have or have had in the past:
- Ocular bacterial or viral infections
- Tendency to suffer from bleeding (e.g., anemia) or stomach ulcers
- Diabetes
- Rheumatoid arthritis
- Dry eye syndrome
- Asthma after using non-steroidal anti-inflammatory drugs
- If you have had recent eye surgery.
- If you have lost sensitivity in the cornea (the transparent layer covering the pupil and iris) or if the normally smooth surface of the cornea is damaged.
Children and Adolescents
ACULAR should not be prescribed for use in children.
Tell your doctor or pharmacist if you are using or have recently used or may need to use any other medication.
If you use ACULAR with any other eye medication, wait at least 5 minutes between the administration of ACULAR and the other medication.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
ACULAR should not be used if you are pregnant or breastfeeding, unless your doctor recommends it.
Driving and Using Machines
ACULAR may cause blurred vision in some patients. Do not drive or use machines until the symptoms have disappeared.
ACULAR contains benzalkonium chloride
This medication contains 0.1 mg of benzalkonium chloride per milliliter, equivalent to 0.1 mg/ml.
Benzalkonium chloride can be absorbed by soft contact lenses, altering their color. Remove contact lenses before using this medication and wait 15 minutes before putting them back.
Benzalkonium chloride can cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer at the front of the eye). Consult your doctor if you feel any strange sensation, itching, or pain in the eye after using this medication.
3. How to use ACULAR
Follow the administration instructions of ACULAR indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again. The recommended dose is one drop in the affected eye, three times a day, for 3-4 weeks after cataract surgery, starting 24 hours before the operation.
Instructions for Use
Apply the eye drops as follows:
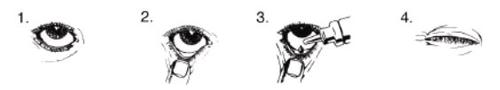
- Wash your hands. Tilt your head back and look up at the ceiling.
- Gently pull down the lower eyelid until a small pocket is formed.
- Turn the container upside down and squeeze it to release one drop into each eye that needs treatment.
- Release the lower eyelid and keep the eye closed for 30 seconds.
If a drop falls outside the eye, repeat the operation.
To avoid contamination or injury, do not allow the tip of the container to touch the eye or any other surface.
Replace the cap and close the container immediately after use.
Wipe any excess liquid from your cheek with a clean cloth.
Correct application of your eye drops is very important.
If you have any doubts about using this medication, ask your doctor or pharmacist.
If you use more ACULAR than you should
Applying an excess of drops is unlikely to produce unwanted side effects. Apply your next dose at the usual time. If someone accidentally ingests this medication, drink liquids to dilute and consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20.
It is recommended to bring the package leaflet and the medication container to the healthcare professional.
If you forget to use ACULAR
If you forget to use ACULAR, apply it as soon as you remember, unless it is almost time for the next dose, in which case skip the missed dose. Apply your next dose and continue with your usual schedule. Do not use a double dose to make up for missed doses.
If you interrupt treatment with ACULAR
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Very Common(may affect more than 1 in 10 people).
- Eye irritation
- itching and/or burning in the eye
- eye pain.
Common(may affect up to 1 in 10 people)
- Allergic reaction
- swelling/inflammation of the eye or eyelid
- itching of the eyes
- red eye
- eye infection
- inflammation of the eye (surface or interior)
- formation of small deposits on the transparent layer at the front of the eye
- retinal bleeding
- inflammation of the central retina (the light-sensitive layer of the eye)
- headache
- accidental injury caused by contact between the dropper and the eye
- increased pressure in the eye
- blurred and/or decreased vision.
Uncommon(may affect up to 1 in 100 people)
- Inflammation or damage to the transparent layer at the front of the eye
- dry eye and/or watery eyes.
Frequency Not Known(frequency cannot be estimated from available data)
- Damage to the surface of the eye, such as thinning
- erosion
- perforation
- degradation of cells
- difficulty breathing or wheezing
- worsening of asthma
- facial inflammation
- ulcer on the surface of the eye.
Side effects related to the cornea (the surface of the eye) may be more likely if you use ACULAR for more than two weeks or if you are using corticosteroid eye drops at the same time or if you have a related eye condition. You should consult your doctor if you experience pain, increased irritation in the eye, and changes in vision.
Reporting Side Effects:
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Conservation of ACULAR
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the label and carton after EXP (which corresponds to the English abbreviation for expiration date). The expiration date is the last day of the month indicated.
Discard the medication 28 days after opening, even if there is still some solution left.
Do not store above 25°C.
Do not use this medication if you notice that the protective seal is broken.
Medications should not be disposed of via wastewater or household waste. Deposit the containers and medications you no longer need at the pharmacy's SIGRE point. Ask your pharmacist how to dispose of containers and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of ACULAR
- The active ingredient is Ketorolaco trometamol 5 mg/ml.
- The other ingredients are benzalkonium chloride, disodium edetate, octoxinol 40, sodium chloride, sodium hydroxide or hydrochloric acid (to adjust the pH) and purified water (see section 2).
Appearance of the Product and Package Contents
ACULAR is a clear to slightly yellowish eye drop solution in a plastic container.
Each container contains 1 plastic bottle of 10 ml capacity with a screw cap.
Each container contains 5 or 10 milliliters of solution.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
AbbVie Spain, S.L.U.
Avenida de Burgos 91
28050, Madrid
Spain
Manufacturer:
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Ireland
This medication is authorized in the Member States of the European Economic Area under the following names:
Austria | ACULAR 0.5% Augentropfen |
Belgium | ACULARE oogdruppels |
Denmark, Ireland, Italy, United Kingdom | ACULAR |
Portugal | ACULAR 0.5% p/v, colírio solução |
Spain | ACULAR 5 mg/ml colirio en solución |
Finland | ACULAR 5 mg/ml eye drops |
France | ACULAR 0.5% |
Greece | ACULAR 0.5% |
Luxembourg | ACULARE collyre |
Netherlands | ACULAR oogdruppels 0.5% |
Germany | ACULAR 5 mg/ml Augentropfen |
Date of the Last Revision of this Leaflet:04/2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
To request a copy of this leaflet in large print, contact the local representative of the marketing authorization holder.
/
- Country of registration
- Average pharmacy price5.18 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACULAR 5 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 1 mg/mlActive substance: diclofenacManufacturer: Laboratoires TheaPrescription requiredDosage form: EYEDROP, 1 mg/mLActive substance: diclofenacManufacturer: Qualix Pharma S.L.Prescription requiredDosage form: EYEDROP, 0.1% sodium diclofenacActive substance: diclofenacManufacturer: Angelini Pharma Espana S.L.Prescription required
Online doctors for ACULAR 5 mg/ml EYE DROPS SOLUTION
Discuss questions about ACULAR 5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















