
ACTIVELLE 1 mg/0.5 mg FILM-COATED TABLETS

How to use ACTIVELLE 1 mg/0.5 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Activelle 1 mg/0.5 mg film-coated tablets
estradiol/norethisterone acetate
Read this package leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Activelle and what is it used for
- What you need to know before you start taking Activelle
- How to take Activelle
- Possible side effects
- Storing Activelle
- Contents of the pack and other information
1. What is Activelle and what is it used for
Activelle is a continuous combined hormone replacement therapy (HRT). It contains two types of female hormones: an estrogen and a progestogen. Activelle is used in postmenopausal women who have had their last natural period at least one year ago.
Activelle is used to:
Relieve symptoms occurring after menopause
During menopause, the amount of estrogen produced by the body decreases. This can cause symptoms such as hot flushes. Activelle relieves these symptoms after menopause. You will only be prescribed Activelle if your symptoms are seriously affecting your daily life.
Prevent osteoporosis
After menopause, some women may have fragile bones (osteoporosis). You should discuss with your doctor what other options are available to you.
If you are at increased risk of getting fractures due to osteoporosis and other medicines are not suitable for you, you can use Activelle to prevent osteoporosis after menopause.
Activelle is prescribed to women who have not had a hysterectomy and whose periods stopped more than one year ago.
There is only limited experience with treating women over 65 years of age with Activelle.
2. What you need to know before you start taking Activelle
Medical history and regular check-ups
The use of HRT carries risks that need to be considered before starting or continuing treatment.
Experience in treating women with premature menopause (due to ovarian failure or surgery) is limited. If you have premature menopause, the risks of HRT may be different. Talk to your doctor.
Before starting (or restarting) HRT, your doctor will ask about your medical history and that of your family. Your doctor may decide to perform a physical examination, which may include a breast examination or an internal examination, if necessary.
Once you start taking Activelle, you will need to have regular check-ups (at least once a year). During these check-ups, you can discuss with your doctor the benefits and risks of continuing Activelle.
Have regular breast examinations as recommended by your doctor.
Do not take Activelle
If any of the following conditions apply to you. If you are unsure about any of the points below, consult your doctor before taking Activelle.
Do not take Activelle:
- If you have, or have had, or are suspected of having breast cancer.
- If you have, or have had, or are suspected of having cancer of the lining of the uterus (endometrial cancer) or another estrogen-dependent cancer.
- If you have any unexplained vaginal bleeding.
- If you have untreated endometrial hyperplasia (a condition where the lining of the uterus grows too much).
- If you have, or have had, a blood clot in a vein (thrombosis), for example, in the legs (deep vein thrombosis) or lungs (pulmonary embolism).
- If you have a blood clotting disorder (such as deficiency of protein C, protein S, or antithrombin).
- If you have, or have had, any blood vessel disease (such as a heart attack, stroke, or angina).
- If you have any liver disease and your liver function tests have not returned to normal.
- If you have a rare blood disorder called porphyria.
- If you are allergic (hypersensitive) to estradiol, norethisterone acetate, or any of the other ingredients of Activelle (listed in section 6, "Contents of the pack and other information").
If any of these conditions occur for the first time while taking Activelle, stop taking it immediately and consult your doctor as soon as possible.
Warnings and precautions
Before starting treatment, tell your doctor if you have ever had any of the following problems, as they may come back or get worse during treatment with Activelle. If so, you will need to have more frequent check-ups:
- fibroids
- endometriosis (a condition where tissue similar to the lining of the uterus grows outside the uterus) or a history of endometrial hyperplasia
- increased risk of blood clots (see "Blood clots in a vein (thrombosis)")
- increased risk of estrogen-sensitive cancer (such as having a mother, sister, or grandmother who has had breast cancer)
- high blood pressure
- a liver disease, such as a benign liver tumor
- diabetes
- gallstones
- migraine or severe headaches
- a disease that affects the immune system, such as systemic lupus erythematosus (SLE)
- epilepsy
- asthma
- a disease that affects the membrane of the eardrum and hearing (otosclerosis)
- a very high level of fat in the blood (triglycerides)
- fluid retention due to heart or kidney problems
- hereditary or acquired angioedema
- lactose intolerance
Stop taking Activelle and inform your doctor immediately
If you notice any of the following symptoms while taking HRT:
- any of the conditions listed in the "Do not take Activelle" section
- yellowing of the skin or whites of the eyes (jaundice), which may be a sign of liver disease
- swelling of the face, tongue, and/or throat, and/or difficulty swallowing or hives, with difficulty breathing, which are signs of angioedema
- a large increase in blood pressure, which may cause headaches, fatigue, dizziness
- migraine-like headaches, which may occur for the first time
- if you become pregnant
- if you notice symptoms of a blood clot, such as:
- inflammation with pain and redness of the legs
- sudden chest pain
- difficulty breathing
For more information, see "Blood clots in a vein (thrombosis)".
Note:Activelle is not a contraceptive. If you are under 50 years old or it has been less than 12 months since your last menstrual period, you may still need to use a contraceptive to prevent pregnancy. Ask your doctor for advice.
HRT and cancer
Endometrial hyperplasia (thickening of the lining of the uterus) and endometrial cancer
Estrogen-only HRT increases the risk of endometrial hyperplasia and endometrial cancer.
The progestogen in Activelle protects against this extra risk.
Irregular bleeding
You may experience irregular bleeding or spotting during the first 3-6 months of taking Activelle.
However, if irregular bleeding:
- continues beyond the first 6 months
- starts after you have been taking Activelle for more than 6 months
- continues after you have stopped taking Activelle
you should talk to your doctor as soon as possible.
Breast cancer
Existing data show that the use of combined estrogen-progestogen HRT or estrogen-only HRT increases the risk of breast cancer. The additional risk depends on how long you take HRT. The additional risk becomes apparent after 3 years of use. After stopping HRT, the additional risk will decrease over time, but the risk may persist for 10 years or more if you have used HRT for more than 5 years.
Comparison
In women aged 50-54 who do not take HRT, 13-17 out of every 1000 will be diagnosed with breast cancer over a 5-year period.
In women aged 50 who start taking estrogen-only HRT for 5 years, there will be between 16 and 17 cases per 1000 women users (i.e., 0-3 extra cases).
In women aged 50 who start taking combined estrogen-progestogen HRT for 5 years, there will be between 21 per 1000 women users (i.e., 4-8 extra cases).
In women aged 50-59 who do not take HRT, an average of 27 cases of breast cancer will be diagnosed per 1000 women over a 10-year period.
In women aged 50 who start taking estrogen-only HRT for more than 10 years, there will be 34 cases per 1000 women users (i.e., 7 extra cases).
In women aged 50 who start taking combined estrogen-progestogen HRT for 10 years, there will be 48 cases per 1000 users (i.e., 21 extra cases).
Examine your breasts regularly. See your doctor if you notice any changes, such as:
- dimpling of the skin
- changes in the nipples
- any lump that you can see or feel
In addition, it is recommended to participate in breast screening programs when they are offered.
During breast screening, it is important to inform your nurse/healthcare professional that you are taking HRT when you have a mammogram, as this medicine may increase the density of your breasts, which can affect the result of the mammogram. When breast density is higher, mammograms may not detect all lumps.
Ovarian cancer
Ovarian cancer is less common than breast cancer. The use of estrogen-only or combined estrogen-progestogen HRT has been associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer varies with age. For example, in women aged 50-54 who do not take HRT, about 2 cases of ovarian cancer will be diagnosed per 2000 women over a 5-year period. In women taking HRT for 5 years, about 3 cases will be diagnosed per 2000 users (i.e., about 1 extra case).
Effect of HRT on the heart and circulation
Blood clots in a vein (thrombosis)
The risk of blood clots in the veins is about 1.3 to 3 times higher in HRT users, especially during the first year.
Blood clots can be serious and, if one travels to the lungs, can cause chest pain, difficulty breathing, fainting, or even death.
The likelihood of a blood clot in the veins will be higher with increasing age and if any of the following apply to you. If any of these situations apply to you, tell your doctor:
- you will be unable to walk for a long time due to a major operation, injury, or illness (see also section 3, "If you need to have surgery")
- you are seriously overweight (body mass index (BMI) >30 kg/m2)
- you have a blood clotting disorder that needs long-term treatment with medication to prevent blood clots
- a close relative has had a blood clot in the legs, lungs, or other organs
- you have systemic lupus erythematosus (SLE)
- you have cancer
To recognize the symptoms of a blood clot, see the section "Stop taking Activelle and inform your doctor immediately".
Comparison
It is estimated that, over a 5-year period, between 4 and 7 out of every 1000 women in their 50s who do not take HRT will have a blood clot in a vein.
In women in their 50s who take combined estrogen-progestogen HRT for 5 years, the number of cases will be between 9 and 12 per 1000 users (i.e., 5 extra cases).
Heart disease (heart attack)
HRT has not been shown to prevent heart attacks. Women over 60 years old who use estrogen-progestogen HRT are slightly more likely to develop heart disease than those who do not.
Stroke
The risk of stroke is about 1.5 times higher in HRT users than in non-users. The number of extra stroke cases due to HRT will increase with age.
Comparison
It is estimated that, over a 5-year period, about 8 out of every 1000 women in their 50s who do not take HRT will have a stroke.
In women in their 50s who take HRT, the number of cases will be about 11 per 1000 users over 5 years (i.e., 3 extra cases).
Other disorders
HRT does not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start HRT after the age of 65. Ask your doctor for advice.
Using Activelle with other medicines
Some medicines may interfere with the effect of Activelle. This can cause irregular bleeding. Such medicines are:
- medicines for epilepsy (such as phenobarbital, phenytoin, and carbamazepine)
- medicines for tuberculosis (such as rifampicin and rifabutin)
- medicines for HIV infection (such as nevirapine, efavirenz, ritonavir, and nelfinavir)
- medicines for hepatitis C infection (such as telaprevir)
- herbal preparations containing St. John's Wort (Hypericum perforatum)
HRT may affect the performance of other medicines:
- epilepsy medicine (lamotrigine), as it may increase the risk of seizures
- hepatitis C medicines (such as the combination of ombitasvir/paritaprevir/ritonavir with or without dasabuvir, as well as a regimen containing glecaprevir/pibrentasvir) may cause increases in liver function blood test results (increase in liver enzyme ALT) in women using combined hormonal contraceptives (CHC) containing ethinylestradiol. Activelle contains estradiol, not ethinylestradiol. It is not known whether liver enzyme ALT may increase when using Activelle with this regimen.
Other medicines may increase the effects of Activelle:
- medicines containing ketoconazole (an antifungal)
Activelle may affect treatment with cyclosporin.
Tell your doctor or pharmacistif you are taking or have recently taken any other medicines, including those obtained without a prescription, herbal medicines, or other natural products. Your doctor will advise you.
Lab tests
If you need to have a blood test, tell your doctor or the laboratory staff that you are taking Activelle, as this medicine may affect the results of some laboratory tests.
Taking Activelle with food and drink
The tablets can be taken with or without food or drink.
Pregnancy and breastfeeding
Pregnancy:Activelle should only be used in postmenopausal women. If you become pregnant, stop taking Activelle and contact your doctor.
Breastfeeding:You should not take Activelle if you are breastfeeding.
Driving and using machines
Activelle has no known effects on the ability to drive or use machines.
Important information about some of the ingredients of Activelle:
Activelle contains lactose monohydrate. If you have an intolerance to certain sugars, consult your doctor before taking Activelle.
3. How to take Activelle
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist. 6
Take one tablet once a day, approximately at the same time every day.When you have finished the 28 tablets in the package, start a new package to continue treatment without interruption.
For more information on using the calendar pack, see the "INSTRUCTIONS FOR USE" section at the end of this prospectus.
You can start treatment with Activelle any day of the week.However, if you have changed from another THS product with which you had menstrual bleeding, start the new treatment right after you have finished the bleeding.
Your doctor should prescribe the lowest effective dose for the shortest possible time that provides relief from symptoms. Talk to your doctor if you think the dose is excessive or insufficient.
If you take more Activelle than you should
Overdose of Activelle can cause dizziness or vomiting. If you take more Activelle than you should, consult your doctor, pharmacist, or call the toxicology information service immediately, telephone 91.562.04.20, indicating the medication and the amount taken. It is recommended to bring the package and prospectus of the medication to the healthcare professional.
If you forget to take Activelle
If you have forgotten to take the corresponding tablet at the usual time, take it within the next 12 hours. If more than 12 hours have passed, skip the missed dose and take it the next day as usual. Do not take a double dose to make up for the missed dose. Forgetting a dose may increase the likelihood of experiencing intercurrent bleeding and spotting, unless you have had a hysterectomy.
If you interrupt treatment with Activelle
If you want to interrupt treatment with Activelle, talk to your doctor first, who will explain the effects of interrupting treatment and discuss other possibilities with you.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
If you are going to have surgery
If you are going to have surgery, inform the surgeon that you are taking Activelle. You may need to stop taking Activelle 4 to 6 weeks before the operation to reduce the risk of blood clots (see section 2, "Blood clots in a vein (thrombosis)"). Ask your doctor when you can start taking Activelle again.
4. Possible side effects
Like all medications, this medication can cause side effects, although not all people experience them.
The following disorders have been reported more frequently in women using THS compared to those who do not use it:
- breast cancer
- abnormal growth or cancer of the uterine lining (endometrial hyperplasia or endometrial cancer)
- ovarian cancer
- blood clots in the veins of the legs or lungs (venous thromboembolism)
- heart disease
- stroke
- possible memory loss if THS is started after 65 years of age.
For more information on these side effects, see the section 2, "What you need to know before taking Activelle".
Hypersensitivity/allergy(uncommon adverse effect - affects 1 to 10 users out of 1000)
Although it is an uncommon effect, hypersensitivity/allergy may occur. The signs of hypersensitivity/allergy may include one or more of the following symptoms: hives, itching, swelling, difficulty breathing, decreased blood pressure (pallor and cooling of the skin, increased heart rate), feeling of dizziness, and sweating that may be signs of an anaphylactic reaction or shock. If one of the mentioned symptoms occurs, stop taking Activelle and seek medical help immediately.
Very common side effects (affect more than 1 in 10 users)
- Pain or tenderness in the breasts
- Vaginal bleeding.
Common side effects (affect 1 to 10 users out of 100)
- Headache
- Weight gain due to fluid retention
- Vaginal inflammation
- Migraine, new onset or worsening of existing
- Vaginal yeast infection
- Depression, new onset or worsening of existing
- Nausea
- Increased size or swelling of the breasts (breast edema)
- Back pain
- Worsening, appearance, or reappearance of uterine fibroids (benign tumor)
- Swelling of arms and legs (peripheral edema)
- Weight gain.
Uncommon side effects (affect 1 to 10 users out of 1000 users)
- Swelling, pain, or discomfort in the abdomen or flatulence
- Acne
- Hair loss (alopecia)
- Abnormal hair growth (male pattern)
- Itching or hives (urticaria)
- Inflammation of a vein (superficial thrombophlebitis)
- Leg cramps
- Pharmacological inefficacy
- Allergic reaction
- Nervousness.
Rare side effects (affect 1 to 10 users out of 10,000)
- Formation of blood clots in the veins of the legs or lungs (deep vein thrombosis, pulmonary embolism).
Very rare side effects (affect less than 1 user out of 10,000)
- Uterine lining cancer (endometrial cancer)
- Excessive thickening of the uterine lining (endometrial hyperplasia)
- Increased blood pressure or worsening of existing hypertension
- Gallbladder disease, gallstones that may be new, recurring, or worsening of existing ones
- Excessive sebum secretion, skin rash
- Acute or recurrent edema (angioneurotic edema)
- Insomnia, dizziness, anxiety
- Changes in sexual desire
- Visual disturbances
- Weight loss
- Vomiting
- Gastric acidity
- Vaginal and genital itching
- Myocardial infarction and stroke.
Other side effects of combined THS
- Gallbladder disease
- Various skin disorders:
- skin pigmentation, especially on the face and neck, known as "pregnancy patch" (chloasma)
- red and painful skin nodules (erythema nodosum)
- rash with ulcers or redness in a target shape (erythema multiforme).
- Red or purple discoloration of the skin and/or mucous membranes (vascular purpura)
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for human use medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Activelle
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the label and on the outer packaging, after "EXP". The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C.
Do not refrigerate.
Keep the package in the outer packaging to protect it from light.
Medications should not be thrown down the drain or into the trash. Deposit the packages and medications you no longer need at the SIGRE  point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packages and medications you no longer need. This way, you will help protect the environment.
point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packages and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Activelle
- The active ingredients are estradiol (as estradiol hemihydrate) 1 mg and norethisterone acetate 0.5 mg.
- The other components are: lactose monohydrate, cornstarch, copovidone, talc, and magnesium stearate.
- The coating contains: hypromellose, triacetin, and talc.
Appearance of the product and package contents
Coated tablets, white, round, with a diameter of 6 mm. On one side, it is engraved "NOVO 288" and on the other side, the Novo Nordisk logo (an Apis bull).
Presentation:
- 1 x 28 coated tablets in a calendar pack
- 3 x 28 coated tablets in calendar packs
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Holder:
Isdin S.A.
Provençals 33
Barcelona 08019
Spain
Manufacturer:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsvaerd
Denmark
This medication is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) with the following names:
Member States of the EEA: Activelle - except in the United Kingdom (Northern Ireland): Kliovance.
Date of the last revision of this prospectus:September 2024
Other sources of information
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http.//www.aemps.gob.es/
INSTRUCTIONS FOR USE
How to use the calendar pack
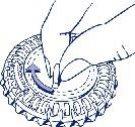
- Place the day indicator
Turn the inner disk and fix the day of the week in front of the closed opening with a plastic tab.
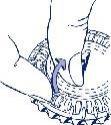
- How to remove the first tablet
Break the plastic tab and remove the first tablet.
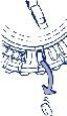
- Move the disk every day
The next day, turn the transparent disk 1 space clockwise, as indicated by the arrow. Remove the next tablet. Remember to take only 1 tablet per day.
The transparent disk can only be turned once the tablet has been removed from the open compartment.
- Country of registration
- Average pharmacy price10.13 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACTIVELLE 1 mg/0.5 mg FILM-COATED TABLETSDosage form: TABLET, Estradiol 0.5 mg / Norethisterone Acetate 0.1 mgActive substance: norethisterone and estrogenManufacturer: Isdin S.A.Prescription requiredDosage form: CAPSULE, 1 mg / 100 mgActive substance: progesterone and estrogenManufacturer: Theramex Ireland LimitedPrescription requiredDosage form: TABLET, 2 mg dienogest; 2 mg estradiol valerateActive substance: dienogest and estrogenManufacturer: Bayer Hispania S.L.Prescription required
Online doctors for ACTIVELLE 1 mg/0.5 mg FILM-COATED TABLETS
Discuss questions about ACTIVELLE 1 mg/0.5 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






