
ACTILOGIC 700 MG MEDICATED ADHESIVE DRESSING

How to use ACTILOGIC 700 MG MEDICATED ADHESIVE DRESSING
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for the user
Actilogic 700 mg medicinal adhesive patch
Lidocaine
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Actilogic and what is it used for
- What you need to know before starting to use Actilogic
- How to use Actilogic
- Possible side effects
- Storage of Actilogic
- Package contents and additional information
1. What is Actilogic and what is it used for
Actilogic contains lidocaine, a local anesthetic, which acts by reducing pain in the skin.
You have been given Actilogic to treat a painful skin problem called postherpetic neuralgia. This problem is usually characterized by localized symptoms such as burning pain, stabbing pain, or shooting pain.
2. What you need to know before starting to use Actilogic
Do not use Actilogic
- if you are allergic to lidocaine or any of the other components of this medicine (listed in section 6),
- if you have had an allergic reaction to other products similar to lidocaine, such as bupivacaine, etidocaine, mepivacaine, or prilocaine,
- on damaged skin or open wounds.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Actilogic.
If you have a severe liver disease, or severe heart problems, or severe kidney problems, you should talk to your doctor before using Actilogic.
Actilogic should only be used on skin areas after the skin has healed completely. It should not be used near the eyes or mouth.
Lidocaine is broken down in the liver into several compounds. One of these compounds is 2,6-xylidine, which has been shown to produce tumors in rats when administered for life at very high doses. The importance of these findings in humans is unknown.
Children and adolescents
Actilogic has not been studied in patients under 18 years of age. Therefore, its use is not recommended in this patient population.
Using Actilogic with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Actilogic should not be used during pregnancy, unless clearly necessary.
No studies have been conducted with the adhesive patch in breastfeeding women. When using Actilogic, only very small amounts of the active ingredient lidocaine may be present in the blood. It is unlikely to have an effect on breastfed babies.
Driving and using machines
It is unlikely that Actilogic will affect your ability to drive or use machines. Therefore, you can drive or operate machines while using Actilogic.
Actilogic contains propylene glycol, methyl parahydroxybenzoate, and propyl parahydroxybenzoate
The patches contain propylene glycol (E1520), a substance that can cause skin irritation. They also contain methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216), substances that can cause allergic reactions. Sometimes, allergic reactions can occur after using the patch for some time.
3. How to use Actilogic
Follow your doctor's instructions for using this medicine exactly. If in doubt, consult your doctor or pharmacist again.
The recommended daily dose consists of covering the painful skin areas using one to three patches. Actilogic can be cut into smaller pieces to fit the affected area. Do not use more than 3 patches at the same time.
The patches should be removed after 12 hours of use, and a 12-hour patch-free period should follow.
You can choose to apply Actilogic during the day or at night.
Usually, you will have some pain relief on the first day you use the patch, but it may take up to 2-4 weeks to see the full effect of Actilogic on pain relief. If after that time you still have a lot of pain, talk to your doctor, as the benefits of treatment should be weighed against the possible risks (see section 2 "Warnings and precautions").
Your doctor will check at regular intervals whether Actilogic is working well.
Before applying Actilogic to the affected area
- If the painful skin area has hair, you can cut the hair using scissors. Do not shave.
- The skin must be clean and dry.
- Creams and lotions can be used on the affected skin during the period when you are not using the patch.
- If you have recently taken a bath or shower, you should wait until the skin cools down before using the patch.
Applying the patch
Step 1:open the envelope and remove one or more patches
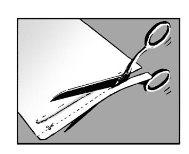
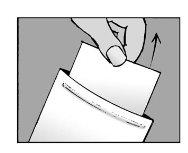
- open the envelope by tearing or cutting along the dotted line
- when using scissors, be careful not to damage the patches
- remove one or more patches depending on the size of the painful skin area
Step 2:close the envelope
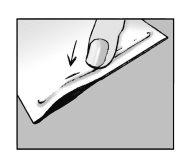
- close the envelope hermetically after use
- the patch contains water and will dry out if the envelope is not closed properly
Step 3:cut the patch, if necessary

- if necessary, cut the patch to fit the size of the painful skin area before removing the protective film
of the adhesive patch
Step 4: remove the protective film
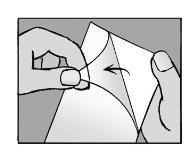
- remove the transparent protective film from the adhesive patch
- try not to touch the adhesive part of the patch
Step 5:apply the patch and press firmly onto the skin
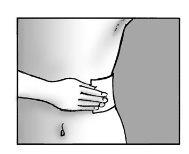
- apply up to three patches to the painful skin area
- press the patch onto the skin
- press for at least 10 seconds to
ensure the patch adheres firmly
- make sure the entire patch adheres to the skin,
including the edges
Leave the adhesive patch on for only 12 hours
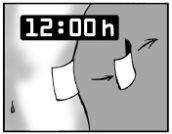
It is essential that Actilogic is in contact with the skin for only 12 hours. For example, if you have more pain at night, you may want to apply the patch at 7 pm and remove it at 7 am.
If you have more pain during the day than at night, you may want to apply Actilogic at 7 am and remove it at 7 pm.
Baths, showers, and swimming
If possible, you should avoid contact with water while using Actilogic. You can bathe, shower, or swim during the time when you are not wearing the patch. If you have recently taken a bath or shower, you should wait until the skin cools down before using the patch.
If the patch comes off
Very rarely, the patch may fall off or come loose. If it does, try to reapply it to the same area. If it does not stick, remove it and apply a new patch to the same area.
How to remove Actilogic
When changing patches, remove the old patch slowly. If it does not come off easily, you can soak it in warm water for a few minutes before removing it.
If you forget to remove the patch after 12 hours
As soon as you remember, remove the old patch. You can use a new patch again after 12 hours.
If you use more patches than you should
If you use more patches than necessary or wear them for too long, this may increase the risk of side effects.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, phone 91 562 04 20.
If you forget to use Actilogic
After the 12-hour patch-free period, if you forget to use a new patch, you should apply a new patch as soon as you remember.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Important side effects or symptoms to look out for and what to do if you are affected:
If irritation or a burning sensation occurs while using the adhesive patch, you should remove the patch. The irritated area should remain patch-free until the irritation stops.
Other side effects that may occur:
Very common(may affect more than 1 in 10 people): Skin problems at or around the patch application site, which may include redness, rash, itching, burning, dermatitis, and small blisters.
Uncommon(may affect up to 1 in 100 people): Skin lesions and skin wounds.
Rare(may affect up to 1 in 10,000 people): Open wound, severe allergic reaction, and allergy.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Actilogic
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the envelope and carton after "EXP". The expiry date is the last day of the month indicated.
Do not refrigerate or freeze.
After first opening: Keep the envelope hermetically sealed.
Validity period after first opening of the envelope: 14 days.
Do not use this medicine if you notice that the envelope has been damaged. If this has happened, the patches may dry out and not adhere properly.
Disposal of Actilogic
Used patches still contain active ingredients, which can be harmful to others. Fold the used patches in half, joining the adhesive sides, and dispose of them in a way that is out of the reach of children.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package contents and additional information
Composition of Actilogic
- The active ingredient is lidocaine.
- Each 14 cm x 10 cm adhesive patch contains 700 mg (equivalent to 5% w/w) of lidocaine.
- The other components of the patch (excipients) are glycerol, liquid sorbitol, sodium carmellose, propylene glycol (E1520), urea, heavy kaolin, tartaric acid, gelatin, polyvinyl alcohol, aluminum glycinates, disodium edetate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), polyacrylic acid, sodium polyacrylate, purified water.
Supporting tissue and release liner: polyethylene terephthalate (PET)
Appearance of the product and package contents
The medicinal patch is 14 cm long and 10 cm wide. It is white and made of non-woven textile material, marked with "Lidocaine 5%". The adhesive patches are packaged in hermetically sealed envelopes, each containing 5 patches.
Each carton contains 5, 10, 20, 25, or 30 patches packaged in 1, 2, 4, 5, or 6 envelopes, respectively. Some pack sizes may not be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Grünenthal Pharma, S.A.
Doctor Zamenhof, 36 – 28027 Madrid
Manufacturer:
Grünenthal GmbH
Zieglerstrasse 6
52078 Aachen
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany | Actilogic |
Spain | Actilogic |
Date of last revision of this leaflet: September 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ACTILOGIC 700 MG MEDICATED ADHESIVE DRESSINGDosage form: GEL/PASTE/ORAL LIQUID, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler & Co GmbhPrescription not requiredDosage form: GEL, 20 mg/mlActive substance: lidocaineManufacturer: Farco-Pharma GmbhPrescription requiredDosage form: CREAM, 40 mg/gActive substance: lidocaineManufacturer: Isdin S.A.Prescription required
Online doctors for ACTILOGIC 700 MG MEDICATED ADHESIVE DRESSING
Discuss questions about ACTILOGIC 700 MG MEDICATED ADHESIVE DRESSING, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















