
ABILIFY MAINTENA 400 mg POWDER AND DILUENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION


How to use ABILIFY MAINTENA 400 mg POWDER AND DILUENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Abilify Maintena 300 mg powder and solvent for prolonged-release injectable suspension
Abilify Maintena 400 mg powder and solvent for prolonged-release injectable suspension
aripiprazole
Read all of this leaflet carefully before you receive this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What Abilify Maintena is and what it is used for
- What you need to know before you are given Abilify Maintena
- How Abilify Maintena is given
- Possible side effects
- Storing Abilify Maintena
- Contents of the pack and other information
1. What Abilify Maintena is and what it is used for
Abilify Maintena contains the active substance aripiprazole and belongs to a group of medicines called antipsychotics. It is used to treat schizophrenia - an illness with symptoms such as hearing, seeing or feeling things that do not exist, mistrust, false beliefs, incoherent speech and emotional and behavioral monotony. People with this illness may also feel depressed, guilty, restless or tense.
Abilify Maintena is indicated for adult patients with schizophrenia who are sufficiently stabilized during treatment with oral aripiprazole.
2. What you need to know before you are given Abilify Maintena
Do not use Abilify Maintena:
- if you are allergic to aripiprazole or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or nurse before you are given Abilify Maintena.
There have been reports of patients experiencing suicidal thoughts and behaviors during treatment with aripiprazole. Tell your doctor immediately if you have thoughts or feelings of harming yourself.
Before treatment with Abilify Maintena, tell your doctor if you suffer from
- an acute state of agitation or an intensely psychotic state.
- heart problems or a history of stroke, especially if you know that you have other risk factors for stroke.
- high blood sugar levels (characterized by symptoms such as excessive thirst, increased urine production, increased appetite, and feeling of weakness) or a family history of diabetes.
- seizures, as your doctor may want to monitor you more closely.
- involuntary and irregular muscle movements, especially in the face.
- a combination of fever, sweating, rapid breathing, muscle stiffness, and drowsiness or numbness (may be signs of neuroleptic malignant syndrome).
- dementia (loss of memory and other mental abilities) especially if you are elderly.
- cardiovascular diseases (heart and circulation diseases), family history of cardiovascular disease, stroke or mini-stroke, abnormal blood pressure.
- irregular heartbeats or if someone else in your family has a history of irregular heartbeats (including the so-called prolongation of the QT interval observed with ECG monitoring).
- blood clots or a family history of blood clots, as antipsychotics have been associated with the formation of blood clots.
- you have any difficulty swallowing.
- a history of addiction to gambling.
- severe liver problems (hepatic).
If you notice that you are gaining weight, developing unusual movements, feeling drowsiness that interferes with your normal daily activities, any difficulty swallowing, or experiencing symptoms of an allergic reaction, talk to your doctor immediately.
Tell your doctor if you, your family, or caregiver notice that you are developing impulses or urges to behave in an unusual way for you and that you cannot resist the impulse, instinct, or temptation to carry out certain activities that may harm you or others. This is called impulse control disorder and may include behaviors such as addiction to gambling, excessive eating or spending, abnormally high sexual appetite, or concern about an increase in sexual thoughts and feelings.
Your doctor may consider adjusting or interrupting the dose.
Aripiprazole may cause drowsiness, a drop in blood pressure when standing up, dizziness, and changes in the ability to move and maintain balance, which could lead to falls. You should be cautious, especially if you are an elderly patient or have some weakness.
Children and adolescents
Do not use this medicine in children and adolescents under 18 years of age. It is not known if it is safe and effective in these patients.
Using Abilify Maintena with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Blood pressure-lowering medicines: Abilify Maintena may increase the effect of medicines used to lower blood pressure. Make sure to tell your doctor if you are taking medicines to control blood pressure.
If you are using Abilify Maintena with another medicine, it may mean that your doctor needs to change your dose of Abilify Maintena or the other medicine. It is especially important that you mention to your doctor if you are taking:
- medicines to correct heart rhythm (such as quinidine, amiodarone, flecainide)
- antidepressants or herbal medicines used to treat depression and anxiety (such as fluoxetine, paroxetine, St. John's Wort)
- medicines to treat fungal infections (antifungals) (such as ketoconazole, itraconazole)
- certain medicines to treat HIV infection (such as efavirenz, nevirapine, and protease inhibitors such as indinavir, ritonavir)
- anticonvulsants used to treat epilepsy (such as carbamazepine, phenytoin, phenobarbital)
- certain antibiotics used to treat tuberculosis (rifabutin, rifampicin)
- medicines that are known to prolong the QT interval.
These medicines may increase the risk of side effects or reduce the effect of Abilify Maintena; if you notice any unusual symptoms when taking any of these medicines at the same time as Abilify Maintena, you should tell your doctor.
Medicines that increase serotonin levels are usually used in diseases that include depression, generalized anxiety disorder, obsessive-compulsive disorder (OCD), and social phobia, as well as migraine and pain:
- triptans, tramadol, and tryptophan used for diseases such as depression, generalized anxiety disorder, obsessive-compulsive disorder (OCD), and social phobia, as well as migraine and pain
- SSRIs (such as paroxetine and fluoxetine) used for depression, OCD, panic, and anxiety
- other antidepressants (such as venlafaxine and tryptophan) used in severe depression
- tricyclic antidepressants (such as clomipramine and amitriptyline) used in depressive illnesses
- St. John's Wort (Hypericum perforatum) used in herbal medicines for mild depression
- analgesics (such as tramadol and pethidine) used to relieve pain
- triptans (such as sumatriptan and zolmitriptan) used to treat migraine.
These medicines may increase the risk of side effects; if you notice any unusual symptoms when taking any of these medicines at the same time as Abilify Maintena, you should tell your doctor.
Using Abilify Maintena with alcohol
Alcohol consumption should be avoided.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use Abilify Maintena if you are pregnantunless you have discussed this with your doctor. Make sure to tell your doctor immediately if you are pregnant, think you may be pregnant, or are planning to become pregnant.
The following symptoms may occur in newborn babies of mothers who have used Abilify Maintena in the last trimester of pregnancy (last three months of pregnancy): tremors, stiffness and/or muscle weakness, drowsiness, agitation, breathing problems, and difficulty feeding. If your baby develops any of these symptoms, you should contact your doctor.
If you are using Abilify Maintena, your doctor will discuss with you whether you should breastfeed your baby, considering the benefit to you of your treatment and the benefit to your baby of breastfeeding. If you are being treated with Abilify Maintena, you should not breastfeed. Talk to your doctor about the best way to feed your baby if you are using Abilify Maintena.
Driving and using machines
During treatment with this medicine, dizziness and vision problems (see section 4) may occur. This should be taken into account when requiring maximum attention, for example, when driving or operating machinery.
Abilify Maintena contains sodium
Abilify Maintena contains less than 1 mmol of sodium (23 mg) per dose. This is essentially 'sodium-free'.
3. How Abilify Maintena is given
Abilify Maintena is presented as a powder, which your doctor or nurse will prepare as a suspension.
Your doctor will decide what dose of Abilify Maintena is suitable for you. The recommended initial dose is 400 mg, unless your doctor decides to give you a lower initial dose or follow-up dose (300 mg, 200 mg, or 160 mg).
There are two ways to start treatment with Abilify Maintena, and your doctor will decide which way is suitable for you.
- If you are given an injection of Abilify Maintena on your first day, treatment with oral aripiprazole will continue for 14 days after the first injection.
- If you are given two injections of Abilify Maintena on your first day, you will also take an oral aripiprazole tablet on this visit.
After that, treatment will be given with Abilify Maintena injections unless your doctor tells you otherwise.
Your doctor will give you the injection as a single injection into the buttock or deltoid (buttock or shoulder) once a month. You may feel some pain during the injection. Your doctor will alternate injections between the right and left sides. Injections will not be given intravenously.
If you are given too much Abilify Maintena
This medicine will be given to you under medical supervision, so it is unlikely that you will be given too much. If you see more than one doctor, make sure to tell them that you are using Abilify Maintena.
Patients who have been given too much aripiprazole have experienced the following symptoms:
- rapid heartbeat, agitation/aggression, speech problems.
- unusual movements (especially of the face or tongue) and decreased level of consciousness
Other symptoms may include:
- acute confusion, seizures (epilepsy), coma, a combination of fever, rapid breathing, sweating,
- muscle stiffness, and drowsiness, slower breathing, choking, high or low blood pressure, abnormal heart rhythms.
Contact your doctor or the nearest hospital immediately if you experience any of the above symptoms.
If you miss a dose of Abilify Maintena
It is important not to forget your scheduled dose. You should receive an injection every month, but not before 26 days have passed since the last injection. If you miss an injection, you should contact your doctor to schedule the next injection as soon as possible.
If administration of Abilify Maintena is stopped
Do not stop your treatment just because you feel better. It is important that you continue to receive Abilify Maintena for the time that your doctor has indicated.
If you have any further questions about the use of this medicine, ask your doctor or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Inform your doctor immediately if you experience any of the following serious adverse effects:
- a combination of any of these symptoms: excessive drowsiness, dizziness, confusion, disorientation, difficulty speaking, difficulty walking, muscle stiffness or tremors, fever, weakness, irritability, aggression, anxiety, elevated blood pressure or convulsions that can lead to loss of consciousness.
- unusual movements mainly of the face or tongue, as your doctor may want to lower the dose.
- a combination of fever, faster breathing, sweating, muscle stiffness, and drowsiness or lethargy, as this can be a sign of a disease called malignant neuroleptic syndrome (NMS).
- more thirst than usual, need to urinate more often, with a lot of appetite, feel weak or tired, feel like vomiting, feel confused or your breath smells like fruit, as this can be a sign of diabetes.
The following adverse effects may also occur after administration of Abilify Maintena.
Frequent Adverse Effects (may affect up to 1 in 10 people):
- weight gain
- diabetes mellitus
- weight loss
- feeling restless
- feeling anxious
- inability to stay still, difficulty staying seated
- sleeping problems (insomnia)
- spasmodic resistance to passive movements such as tensing and relaxing muscles, increased muscle tone in an abnormal way, slow body movements
- akathisia (an uncomfortable feeling of inner restlessness and an urgent need to move constantly)
- seizures or tremors
- uncontrollable tics, twitches, or twisting movements
- changes in level of awareness, numbness
- drowsiness
- dizziness
- headache
- dry mouth
- muscle stiffness
- inability to have or maintain an erection during sexual intercourse
- pain at the injection site, skin hardening at the injection site
- weakness, loss of strength, or extreme fatigue
- during blood tests, your doctor may find elevated levels of creatine phosphokinase in the blood (an enzyme important for muscle function)
Uncommon Adverse Effects (may affect up to 1 in 100 people):
- low levels of a certain type of white blood cell (neutropenia), low hemoglobin, or low red blood cell count, low platelet count in the blood
- allergic reaction (hypersensitivity)
- increased or decreased levels of the hormone prolactin in the blood
- increased blood sugar
- increased blood fats such as cholesterol, elevated triglycerides, and also low cholesterol and low triglyceride levels
- increased insulin levels, a hormone that regulates blood sugar levels
- increased or decreased appetite
- suicidal thoughts
- mental disorder characterized by defective perception or loss of reality
- hallucinations
- delirium
- increased sexual interest
- panic attack
- depression
- emotional instability
- state of indifference with lack of emotion, feelings of emotional and mental distress
- sleep disorder
- grinding teeth or clenching the jaw
- decreased sexual interest (decreased libido)
- altered mood
- muscle problems
- uncontrollable muscle movements, such as grimacing, lip smacking, or tongue movements. These usually affect the face and mouth first but can affect other parts of the body. These can be signs of a disorder called "tardive dyskinesia".
- parkinsonism: a condition with many and varied symptoms that include slow or reduced movements, slow thinking, jerking when bending limbs (cogwheel rigidity), shuffling feet, rapid steps, tremors, scarce or absent facial expression, muscle stiffness, drooling
- movement problems
- extreme restlessness and restless legs
- distortion of the senses of taste and smell
- fixation of the eyeballs in a position
- blurred vision
- eye pain
- double vision
- ocular photosensitivity
- abnormal heartbeat, rapid or slow heartbeat, abnormal electrical conduction of the heart, abnormal electrocardiogram (ECG)
- high blood pressure
- dizziness when standing up after being lying down or sitting due to a drop in blood pressure
- cough
- hiccups
- gastroesophageal reflux disease. Excessive amount of gastric juice that backs up (reflux) into the esophagus (throat or the tube that goes from the mouth to the stomach through which food passes), causing stomach acid and possibly damaging the esophagus
- heartburn
- vomiting
- diarrhea
- nausea
- stomach pain
- stomach discomfort
- constipation
- frequent bowel movements
- drooling, more saliva in the mouth than normal
- abnormal hair loss
- acne, skin disease where the nose and cheeks are unusually reddened, eczema, skin hardening
- muscle stiffness, muscle spasms, muscle tics, muscle tension, muscle pain (myalgia), pain in the limbs
- joint pain (arthralgia), back pain, decreased joint mobility, stiff neck, limited mouth opening
- kidney stones or sugar (glucose) in the urine
- spontaneous milk secretion from the breasts (galactorrhea)
- increased breast size in men, painful breasts, vaginal dryness
- fever
- loss of strength
- gait disturbance
- chest discomfort
- injection site reactions such as redness, swelling, discomfort, and itching at the injection site
- thirst
- slowness
- liver function tests may show abnormal results
- during tests, your doctor may find:
- elevated levels of liver enzymes
- elevated levels of alanine aminotransferase
- elevated levels of γ-glutamyl transferase
- elevated levels of bilirubin in your blood
- elevated levels of aspartate aminotransferase
- elevated or reduced glucose levels in the blood
- elevated levels of glycosylated hemoglobin
- reduced cholesterol levels in the blood
- reduced triglyceride levels in the blood
- increased waist circumference
The following adverse effects have been reported since the marketing of oral aripiprazole, but the frequency with which they occur is unknown (the frequency cannot be estimated from the available data):
- low levels of white blood cells
- allergic reaction (e.g., swelling of the mouth, tongue, face, and throat, itching, hives), rash
- abnormal heartbeat, sudden unexplained death, heart attack
- diabetic ketoacidosis (ketones in the blood and urine) or coma
- loss of appetite (anorexia), difficulty swallowing
- low sodium levels in the blood
- suicide attempt and completed suicide
- inability to resist the impulse, instinct, or temptation to perform an action that may be harmful to you or others, which may include:
- strong impulse to gamble excessively despite serious personal or family consequences
- altered or increased sexual interest and worrisome behavior for you or others, for example, increased sexual appetite
- uncontrollable excessive shopping
- binge eating (eating large amounts of food in a short period) or compulsive eating (eating more food than normal and more than needed to satisfy hunger)
- tendency to wander
Inform your doctor if you experience any of these behaviors; he will explain how to manage or reduce the symptoms.
- nervousness
- aggression
- malignant neuroleptic syndrome (a syndrome with symptoms such as fever, muscle stiffness, accelerated breathing, sweating, decreased consciousness, and sudden changes in blood pressure and heart rate)
- seizures (attacks)
- serotonin syndrome (a reaction that can cause a feeling of intense happiness, drowsiness, clumsiness, restlessness, feeling of being drunk, fever, sweating, muscle stiffness)
- speech disorders
- heart problems, including ventricular helicoidal tachycardia, cardiac arrest, irregularities in heart rhythm that may be due to abnormal nerve impulses in the heart, abnormal electrocardiogram (ECG) readings, prolonged QT interval
- fainting
- symptoms related to blood clots in the veins, especially in the legs (symptoms include swelling, pain, and redness in the leg), which can travel through the blood vessels to the lungs, causing chest pain and difficulty breathing
- spasm of the muscles around the glottis
- accidental aspiration of food with risk of pneumonia (lung infection)
- pancreatitis
- difficulty swallowing
- liver failure
- jaundice (yellow color of the skin and the white part of the eyes)
- liver inflammation
- rash
- skin photosensitivity
- excessive sweating
- severe allergic reactions, such as drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). DRESS syndrome initially appears as pseudogripal symptoms with a rash on the face and, later, with prolonged rash, high temperature, enlarged lymph nodes, increased liver enzyme concentrations observed in blood tests, and increased levels of a type of white blood cell (eosinophilia)
- muscle weakness, sensitivity, or pain, and particularly if you feel unwell, have a high temperature, or have dark urine. These can be caused by abnormal muscle metabolism that is potentially fatal and can cause kidney problems (a condition called rhabdomyolysis)
- difficulty urinating
- involuntary loss of urine (incontinence)
- withdrawal symptoms in newborns
- prolonged and/or painful erection
- difficulty controlling body temperature or overheating
- chest pain
- swelling of the hands, ankles, or feet
- during tests, your doctor may find:
- elevated levels of alkaline phosphatase
- fluctuating results during glucose tests in the blood
Reporting of Adverse Effects
If you experience adverse effects, consult your doctor or nurse, even if they are adverse effects that do not appear in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Abilify Maintena
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and vial. The expiration date is the last day of the month indicated.
Do not freeze.
The reconstituted suspension should be used immediately but can be stored in the vial for 4 hours at a temperature below 25 °C. Do not store the reconstituted suspension in the syringe.
6. Container Contents and Additional Information
Composition of Abilify Maintena
- The active ingredient is aripiprazole.
Each vial contains 300 mg of aripiprazole.
After reconstitution, each ml of suspension contains 200 mg of aripiprazole. Each vial contains 400 mg of aripiprazole.
After reconstitution, each ml of suspension contains 200 mg of aripiprazole.
- The other components are
Powder
Sodium carmellose, mannitol, sodium phosphate monohydrate, sodium hydroxide
Solvent
Water for injectable preparations
Appearance of Abilify Maintena and Container Contents
Abilify Maintena is a powder and solvent for the preparation of a prolonged-release injectable suspension.
Abilify Maintena is a white or off-white powder presented in a transparent glass vial. Your doctor or nurse will prepare an Abilify Maintena suspension that will be administered to you in an injection. To do this, they will use the vial of solvent that comes in the pack, a clear solution in a transparent glass vial.
Single Pack
Each single pack contains a vial with powder, a 2 ml vial with solvent, a 3 ml luer-lock syringe with a pre-attached 38 mm, 21-gauge safety needle, with a needle protection device, a 3 ml luer-lock disposable syringe, a vial adapter, and three safety needles: one 25 mm, 23-gauge, one 38 mm, 22-gauge, and one 51 mm, 21-gauge.
Multipack
Pack of 3 single packs.
Only certain pack sizes may be marketed.
Marketing Authorisation HolderOtsuka Pharmaceutical Netherlands B.V. Herikerbergweg 292
1101 CT, Amsterdam
Netherlands
Manufacturer
- Lundbeck A/S Ottiliavej 9, 2500 Valby
Denmark
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Lundbeck S.A./N.V. Tél/Tel: +32 2 535 79 79 | Lietuva
Tel: +45 36301311 |
България Lundbeck Export A/S Representative Office Tel: +359 2 962 4696 | Luxembourg/Luxemburg Lundbeck S.A. Tél: +32 2 535 79 79 |
Česká republika Lundbeck Česká republika s.r.o. Tel: +420 225 275 600 | Magyarország Lundbeck Hungaria Kft. Tel: +36 1 4369980 |
Danmark Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 | Malta
Tel: +45 36301311 |
Deutschland Otsuka Pharma GmbH Tel: +49 69 1700860 | Nederland Lundbeck B.V. Tel: +31 20 697 1901 |
Eesti
Tel: +45 36301311 | Norge Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 |
Ελλάδα Lundbeck Hellas S.A. Τηλ: +30 210 610 5036 | Österreich Lundbeck Austria GmbH Tel: +43 1 266 91 08 |
España Otsuka Pharmaceutical S.A. Tel: +34 93 208 10 20 | Polska Lundbeck Poland Sp. z o. o. Tel.: +48 22 626 93 0 |
France Otsuka Pharmaceutical France SAS Tél: +33 (0) 1 47 08 00 00 | Portugal Lundbeck Portugal Lda Tel: +351 21 00 45 900 |
Hrvatska Lundbeck Croatia d.o.o. Tel.: +385 1 644 82 63 | România Lundbeck Export A/S Reprezentanta din Romania Tel: +40 21319 88 26 |
Ireland Lundbeck (Ireland) Limited Tel: +353 1 468 980 | Slovenija Lundbeck Pharma d.o.o. Tel.: +386 2 229 4500 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Lundbeck Slovensko s.r.o. Tel: +421 2 5341 42 18 |
Italia Otsuka Pharmaceutical Italy S.r.l Tel: +39 02 00 63 27 10 | Suomi/Finland Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 |
Κύπρος Lundbeck Hellas A.E Τηλ.: +357 22490305 | Sverige Otsuka Pharma Scandinavia AB Tel: +46 8 54528660 |
Latvija
Tel: +45 36301311 | United Kingdom Otsuka Pharmaceuticals (UK) Ltd. Tel: +44 203 747 5300 |
Date of Last Revision of this Leaflet: {MM/YYYY}.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency web site http://www.ema.europa.eu.
-----------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
Disposal of unused medicinal product and all materials that have come into contact with it should be done in accordance with local regulations.
Abilify Maintena 300 mg powder and solvent for prolonged-release injectable suspension
Abilify Maintena 400 mg powder and solvent for prolonged-release injectable suspension
Aripiprazole
Step 1: Preparation before reconstituting the powder
Remove all components from the pack and confirm that they are all as listed below:
- Abilify Maintena package leaflet and instructions for healthcare professionals
- Vial with powder
- Vial with 2 ml of solvent
Important:the vial with solvent contains an additional amount of solvent.
- A 3 ml luer-lock syringe with a pre-attached 38 mm, 21-gauge safety needle, with a needle protection device
- A 3 ml disposable syringe with luer-lock tip
- A vial adapter
- A 25 mm, 23-gauge safety needle with needle protection device
- A 38 mm, 22-gauge safety needle with needle protection device
- A 51 mm, 21-gauge safety needle with needle protection device
- Instructions for the needle and syringe
Step 2: Reconstitution of the powder
- Remove the cap from the vial with powder and the vial with solvent, and clean the stopper of both vials with a sterile alcohol swab.
- Using the syringe with the pre-attached needle, withdraw the predetermined volume of solvent from the vial, drawing the solvent into the syringe.
Vial of 300 mg:
Add 1.5 ml of solvent to reconstitute the powder.
Vial of 400 mg:
Add 1.9 ml of solvent to reconstitute the powder.
A small amount of residual solvent will remain in the vial after withdrawal. Any excess should be discarded.

Water
- Slowly inject the solvent into the vial containing the powder.
- Withdraw the air to equalize the pressure in the vial by gently pulling back the plunger.

- Then remove the syringe from the vial.
Proceed to engage the needle safety device using a one-handed technique.
Gently press the sheath against a flat surface until the needle is firmly seated in the needle protection sheath.
Visually confirm that the needle is fully engaged in the needle protection sheath and discard the syringe.

- Shake the vial vigorously for at least 30 seconds until the suspension appears uniform.
- Visually inspect the reconstituted suspension for particles and discoloration before administration. The reconstituted medicinal product is a white or off-white liquid suspension. Do not use the reconstituted suspension if it contains particles or is discolored.
- If the injection is not administered immediately after reconstitution, the vial can be stored for up to 4 hours at a temperature below 25°C and shaken vigorously for at least 60 seconds to ensure re-suspension before injection.
- Do not store the reconstituted suspension in the syringe.
Step 3: Preparation before injection
- Remove the protective cover from the vial adapter, but do not remove the vial adapter from the protective cover.
- Hold the protective cover of the vial adapter and twist the luer-lock syringe into the vial adapter.

- Use the luer-lock syringe to remove the vial adapter from the protective cover and discard the protective cover of the vial adapter. Do not touch the tip of the vial adapter at any time.

- Determine the recommended volume for injection.
Abilify Maintena vial of 300 mg | |
Dose | Volume to inject |
--- | --- |
300 mg | 1.5 ml |
200 mg | 1.0 ml |
160 mg | 0.8 ml |
Abilify Maintena vial of 400 mg | |
Dose | Volume to inject |
400 mg | 2.0 ml |
300 mg | 1.5 ml |
200 mg | 1.0 ml |
160 mg | 0.8 ml |
- Clean the stopper of the vial with the reconstituted suspension with a sterile alcohol swab.
- Place and hold the vial with the reconstituted suspension on a hard surface. Engage the vial adapter by holding the outer part of the vial adapter and pushing the tip of the vial adapter firmly through the rubber stopper until it clicks into place.
- Slowly withdraw the recommended volume from the vial with the luer-lock syringe for injection. A small amount of suspension will remain in the vial.

Step 4: Injection procedure
- Remove the luer-lock syringe containing the recommended volume of reconstituted Abilify Maintena suspension from the vial.
- Select one of the safety needles described below, based on the injection site and patient weight, and attach the safety needle to the luer-lock syringe containing the suspension for injection. Ensure the safety needle is firmly seated on the needle protection device by twisting in a clockwise direction, then remove the needle cover immediately.
Morphological Type | Injection Site | Needle Size |
Non-obese | Deltoid | 25 mm, 23-gauge |
Gluteal | 38 mm, 22-gauge | |
Obese | Deltoid | 38 mm, 22-gauge |
Gluteal | 51 mm, 21-gauge |
- Inject the recommended volume slowly as a single dose via intramuscular injection into the gluteal or deltoid muscle. Do not massage the injection site. Care should be taken to avoid accidental intravascular injection. Do not inject into areas with signs of inflammation, damaged skin, swelling, and/or hematoma.
For deep intramuscular injection into the gluteal or deltoid muscle only.
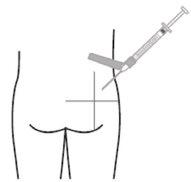
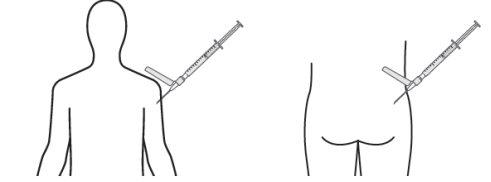
Deltoid Gluteal
Remember to rotate the injection sites in both gluteal and deltoid muscles. If treatment is initiated with two injections, administer the injections at two different sites in two different muscles. DO NOT inject both injections together in the same deltoid or gluteal muscle.
In the case of known CYP2D6 poor metabolizers, administer in two separate deltoid muscles or in one deltoid muscle and one gluteal muscle. DO NOT inject into two gluteal muscles.
Monitor for signs or symptoms of unintentional intravenous administration.
Step 5: Procedures after injection
Engage the needle safety device as described in Step 2 e). Discard the vials, adapter, needles, and syringe in an appropriate manner after injection. The powder and solvent vials are for single use only.

- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ABILIFY MAINTENA 400 mg POWDER AND DILUENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: ORAL SOLUTION/SUSPENSION, 1 mg/mlActive substance: aripiprazoleManufacturer: Kern Pharma S.L.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 10 mgActive substance: aripiprazoleManufacturer: Kern Pharma S.L.Prescription requiredDosage form: TABLET, 10 mgActive substance: aripiprazoleManufacturer: Kern Pharma S.L.Prescription required
Online doctors for ABILIFY MAINTENA 400 mg POWDER AND DILUENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about ABILIFY MAINTENA 400 mg POWDER AND DILUENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











