SONDELBAY 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen


How to use SONDELBAY 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Sondelbay 20 micrograms/80 microliters solution for injection in pre-filled pen
teriparatide
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Sondelbay and what is it used for
- What you need to know before you use Sondelbay
- How to use Sondelbay
- Possible side effects
- Storage of Sondelbay
- Contents of the pack and other information
1. What is Sondelbay and what is it used for
Sondelbay contains the active substance teriparatide, which is used to increase bone strength and reduce the risk of fractures by stimulating bone formation.
Sondelbay is used for the treatment of osteoporosis in adults. Osteoporosis is a disease that makes your bones waste away and become fragile. This disease is especially common in women after menopause, but it can also occur in men. Osteoporosis is also common in patients treated with corticosteroids.
2. What you need to know before you use Sondelbay
Your healthcare professional will teach you how to use the Sondelbay pen.
Do not use Sondelbay
- if you are allergic to teriparatide or any of the other ingredients of this medicine (listed in section 6).
- if you have high levels of calcium (pre-existing hypercalcemia).
- if you have severe kidney problems.
- if you have ever been diagnosed with bone cancer or other types of cancer that have spread (metastasized) to your bones.
- if you have certain bone diseases. If you have a bone disease, consult your doctor. if you have high levels of alkaline phosphatase in your blood without apparent explanation, which could indicate that you have Paget's disease of the bone (a disease with abnormal bone changes). If you are not sure, consult your doctor.
- if you have received radiation therapy that may have affected your bones.
- if you are pregnant or breastfeeding.
Warnings and precautions
Sondelbay may cause an increase in the amount of calcium in your blood or urine.
Consult your doctor or pharmacist before starting or while using Sondelbay:
- if you constantly have nausea, vomiting, constipation, low energy, or muscle weakness, tell your doctor. These may be symptoms of too much calcium in your blood.
- if you have kidney stones or have a history of kidney stones.
- if you have kidney problems (moderate renal insufficiency), you should tell your doctor.
Some patients, after the first doses, may experience dizziness or an increase in heart rate. For the first doses, use Sondelbay in a place where you can sit or lie down immediately if you feel dizzy.
The recommended treatment time of 24 months should not be exceeded. Sondelbay should not be used in growing adults.
Children and adolescents
Sondelbay should not be used in children and adolescents (under 18 years of age).
Other medicines and Sondelbay
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, because occasionally interactions may occur (e.g., digoxin/digitalis, a medicine used to treat heart diseases).
Pregnancy and breastfeeding
Do not use Sondelbay if you are pregnant or breastfeeding. If you are a woman of childbearing age, you should use effective contraceptive methods during treatment with Sondelbay. If you become pregnant, treatment with Sondelbay should be discontinued. Consult your doctor or pharmacist before using this medicine.
Driving and using machines
Some patients may feel dizzy after the injection of Sondelbay. If you feel dizzy, do not drive or use machines until you feel better.
Sondelbay contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially “sodium-free”.
3. How to use Sondelbay
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is 20 micrograms (in 80 microliters) administered once a day by subcutaneous injection in the thigh or abdomen. To help you remember to inject your medicine, inject it at the same time every day.
Inject Sondelbay every day for as long as your doctor prescribes it. The total duration of treatment with Sondelbay should not exceed 24 months. You should not receive more than one 24-month treatment cycle with Sondelbay throughout your life.
See the instructions for use on how to use the Sondelbay pen.
No needles are included with the pen. Pen needles (31G or 32G; 4mm, 5mm, or 8mm) may be used.
The injection of Sondelbay should be performed shortly after removing the pen from the refrigerator, as indicated in the User Manual. Return the pen to the refrigerator immediately after use. You should use a new needle for each injection and discard it after each use. Do not store the pen with the needle attached. Never share your Sondelbay pen with others.
Your doctor may recommend that you take calcium and vitamin D with Sondelbay. Your doctor will tell you how much to take each day.
Sondelbay can be used with or without food.
If you use more Sondelbay than you should
If you have accidentally administered more Sondelbay than prescribed, consult your doctor or pharmacist.
The effects that could be expected from an overdose include nausea, vomiting, dizziness, and headache.
If you forget or are unable to inject Sondelbay at the usual time, do so as soon as possible on the same day. Do not administer a double dose to make up for forgotten doses. Do not inject more than once on the same day. Do not try to make up for the forgotten dose.
If you stop treatment with Sondelbay
If you are thinking of stopping treatment with Sondelbay, please consult your doctor. Your doctor will advise and decide how long you should be treated with Sondelbay.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects are pain in the limbs (very common, may affect more than 1 in 10 patients), discomfort, headache, and dizziness (common). If you feel dizzy after an injection, sit or lie down until you feel better. If it does not improve, consult your doctor before continuing treatment. Cases of fainting associated with the use of teriparatide have been reported.
If you experience discomfort such as redness of the skin, pain, swelling, itching, bruising, or slight bleeding at the injection site (common), these should disappear within a few days or weeks. If not, tell your doctor as soon as possible.
Some patients may have experienced allergic reactions just after injection, consisting of difficulty breathing, swelling of the face, skin rash, and chest pain (rare). In rare cases, severe and potentially life-threatening allergic reactions, including anaphylaxis, may occur.
Other side effects are:
Common: may affect up to 1 in 10 patients
- increase in blood cholesterol levels
- depression
- neuralgic pain in the leg
- feeling of fainting
- irregular heartbeats
- difficulty breathing
- increased sweating
- muscle cramps
- loss of energy
- fatigue
- chest pain
- low blood pressure
- stomach acid (pain or burning sensation just below the breastbone)
- vomiting
- hernia of the tube that carries food to your stomach
- low hemoglobin or low red blood cell count (anemia)
Uncommon: may affect up to 1 in 100 patients
- increased heart rate
- abnormal heart sound
- shortness of breath
- hemorrhoids
- accidental loss or leakage of urine
- increased need to urinate
- weight gain
- kidney stones
- pain in the muscles and joints. Some patients have experienced severe back cramps or pain and had to be hospitalized.
- increase in blood calcium levels
- increase in blood uric acid levels
- increase in the levels of an enzyme called alkaline phosphatase.
Rare: may affect up to 1 in 1,000 patients
- reduction of kidney function, including renal failure
- swelling, mainly in the hands, feet, and legs.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sondelbay
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and pen after CAD and EXP respectively. The expiry date is the last day of the month stated.
Sondelbay should always be stored in a refrigerator (between 2°C and 8°C). Once opened, Sondelbay can be stored at temperatures up to 25°C for a maximum of three days when no refrigeration is available. After this period, the medicine should be returned to a refrigerator and used within 28 days of the first injection. The Sondelbay pen should be discarded if it has been out of the refrigerator at temperatures up to 25°C for more than three days.
Do not freeze Sondelbay. Avoid placing the pens near the freezer compartment of the refrigerator to prevent freezing. Do not use Sondelbay if it is or has been frozen.
Store in the original package (the outer cardboard box) to protect it from light.
Each pen should be discarded after 28 days, even if it is not empty.
Sondelbay contains a clear and colorless solution. Do not use Sondelbay if it has solid particles or if the solution is cloudy or discolored.
Never transfer the medicine to a syringe.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
- The active substance is teriparatide. Each milliliter of solution for injection contains 250 micrograms of teriparatide. Each 80-microliter dose contains 20 micrograms of teriparatide. A 2.4-ml pre-filled pen contains 600 micrograms of teriparatide.
- The other ingredients are glacial acetic acid, sodium acetate (anhydrous), mannitol, metacresol, and water for injections. Additionally, hydrochloric acid and/or sodium hydroxide solution may have been added to adjust the pH (see section 2 “Sondelbay contains sodium”).
Appearance of the product and pack contents
Sondelbay is a clear and colorless solution. It is presented in a cartridge contained in a disposable pre-filled pen. Each pre-filled pen contains 2.4 ml of solution sufficient for 28 doses. Sondelbay is available in packs containing one or three pre-filled pens. Some pack sizes may only be available.
Marketing authorization holder
Accord Healthcare S.L.U.
World Trade Centre,
Moll de Barcelona s/n, Edifici Est, 6ª Planta,
08039, Barcelona, Spain
Manufacturer
Accord Healthcare BV,
Netherlands Winthontlaan 200,
Utrecht, 3526KV, Netherlands
Accord Healthcare Polska Sp.z o.o.,
ul. Lutomierska 50,
95-200 Pabianice, Poland
Date of last revision of this leaflet:
Detailed information on this medicine is available on the European Medicines Agency website http://www.ema.europa.eu/
Sondelbay20 micrograms/80 microliters solution for injection in pre-filled pen teriparatide
Instructions for use
Before using your new pen, please read both sides of these Instructions for use in their entirety. On the back, you will find information on troubleshooting and additional data.
Follow these instructions carefully each time you use the Sondelbay pen. Also, read the package leaflet provided in the package, with information for the patient.
Do not share the Sondelbay pen or its needles with others, as this may pose a risk of transmission of infections or other conditions.
Your pen contains medicine for 28 days.
Discard the Sondelbay pen after 28 days of the first injection, even if the cartridge is not completely empty.
Do not inject more than one dose of Sondelbay on the same day.
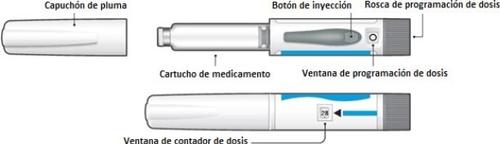
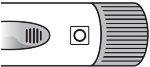
Parts of the Sondelbay pen | ||
| ||
Needles are not included |
| |
| Checkthe dose counter window to determine the number of doses remaining. The needle indicatesthe number of remaining doses. A new pen should contain 28 doses. The black dots that appear in the dose counter window indicatethe odd dose numbers remaining in the |
It should be used with pen needles (31G or 32G; 4mm, 5mm, or 8mm). Ask your pharmacist for the ideal needle gauge and length for you. Use a new needle for each injection. | pen. Do not use the pen if the counter indicates '00' as this means that there are no doses left. The Sondelbay pen does not need to be primed. |
1 Preparation |
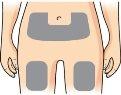
Prepare the injection site (thigh or abdomen) according to your doctor's or pharmacist's instructions. |
A new pen should contain 28 doses. |
Remove the pen cap. |
|
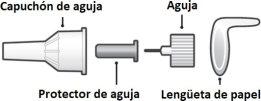
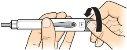
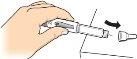
2 Place a new needle |
Find a new needle (see previous section). Remove the paper tab. |
Place the needle at pressure directlyonto the medication cartridge. |
Screwthe needle until it is firmlysecured. |
Remove the needle cap and saveit. You will need it to remove the needle after use. |
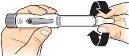
3 Program the dose |
Checkthat an empty circle appears in the dose programming window. |
Turnthe dose programming screw to the right; arrows appear in the dose programming window. |
Keep turning until you hear a “click” and see a black circlein the dose programming window. |
Release the dose programming screw. In the window, the black circle with a bar above appears. This confirms that the dose has been programmed. |
Removethe needle protector and discardit. |
4 Inject the dose |
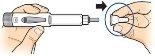
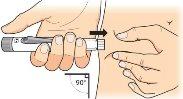
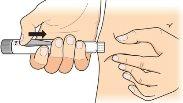
Gently pinch the skin of the thigh or abdomen and insert the needle directly into the skin, making sure to see the dose programming window. dose. |
Keep the needle in the skin, pressthe injection button until it stops. This starts the injection. |
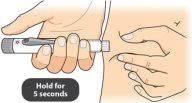
Keep the needle in the skin and wait until an empty circle appears in the dose programming window. Then slowly count to 5,and remove the needle from the skin. |
5 Confirm the dose |
| Once the injection is completeand the needle is removed from the skin, make surethat an empty circle appears in the dose programming window. | If the empty circle does not appearin the dose programming window, do not inject againon the same day. Instead, you will need to reprogram the pen. See section D of Troubleshooting. |
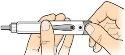
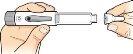
6 Remove the needle |
Place the needle cap over the needle, as indicated, and then pushit to secure it in place. To avoid needlestick injuries, never attemptto put the needle protector back on, nor touch the needle. |
Turn the needle cap to the left at least 5 times to unscrew it from the pen. |
Removethe needle and discard it according to your doctor's or pharmacist's instructions. |
Put the cap back on the pen and secure it firmly. Immediately after use, store the pen in the refrigerator. |
Troubleshooting | ||
Problem | Solution | |
A. | I can see an air bubble in the Sondelbay pen. | A small air bubble will not affect the dose, nor will it harm you. You can continue to administer the dose as usual. |
B. | I cannot administer the dose. |
|
C. | I can see a drop of medication on the tip of the needle when I remove the needle protector to administer the injection. | A small drop of medication on the tip of the needle will not affect the dose. Continue to administer the dose as described in Step 4 of the Instructions for use. |
D. | The empty circle did not appear in the dose programming window, even after pressing the injection button to the maximum and waiting. What should I do? | To reprogram the Sondelbay pen, you should follow the steps below:
Do nottouch the needle. Do not attemptto put the needle protector back on. Unscrew the needle and discard it according to your doctor's or pharmacist's instructions.
Do not attemptto put the needle protector back on. |
Unscrew the needle and discard it according to your doctor's or pharmacist's instructions.
This problem can be avoided if you always use a NEW needle for each injection, and also by pressing the injection button to the maximum. Wait until the empty circle appears and then count to 5 before removing the needle from the skin. | ||
E. | How can I know that my Sondelbay pen is working properly? | The Sondelbay pen is designed to inject a complete dose each time it is used according to the Instructions for use. After injection, the empty circle appears in the dose programming windowto indicate that the complete dose of medication has been administered. The dose counter windowindicates the number of doses remaining in the pen. It shows the countdown each time a dose is administered. This also indicates that the pen is working properly. Use a new needle for each injection to ensure that the Sondelbay pen works correctly. |
F. | I cannot remove the needle from the Sondelbay pen. |
|
Cleaning and maintenance |
Cleaning your Sondelbay pen:
Storing the Sondelbay pen:
|
Disposal of the Sondelbay pen and needles |
Disposal of the Sondelbay pen
|

Additional information |
|
Date of the last revision of this manual:
- Country of registration
- Average pharmacy price252.16 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SONDELBAY 20 micrograms/80 microliters Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 250 micrograms/mlActive substance: teriparatideManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 250 µg/mlActive substance: teriparatideManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, 20 micrograms/80 microlitersActive substance: teriparatideManufacturer: Theramex Ireland LimitedPrescription required
Online doctors for SONDELBAY 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen
Discuss questions about SONDELBAY 20 micrograms/80 microliters Injectable Solution in Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions