
SILOCALM 1 mg/ml ORAL SUSPENSION

How to use SILOCALM 1 mg/ml ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Silocalm 1 mg/ml Oral Suspension
clobazam
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Pack
- What Silocalm is and what it is used for
- What you need to know before you take Silocalm
- How to take Silocalm
- Possible side effects
- Storage of Silocalm
- Contents of the pack and other information
1. What Silocalm is and what it is used for
Silocalm oral suspension contains clobazam, which belongs to a group of medicines called benzodiazepines.
Clobazam has a calming effect on the brain.
Silocalm oral suspension is used for the treatment of:
- Epilepsy (seizures) (in combination with other treatments) in adults or children over 2 years of age if it is not controlled with conventional treatment with one or more anticonvulsants.
2. What you need to know before you take Silocalm oral suspension
Do not take Silocalm oral suspension:
- If you are allergic (hypersensitive) to clobazam, other medicines of the benzodiazepine group, or any of the other ingredients of this medicine (listed in section 6).
The symptoms of an allergic reaction include: skin rash, difficulty swallowing or breathing, swelling of the lips, face, throat, or tongue.
- If you have a disease that causes muscle weakness (called 'myasthenia gravis')
- If you have breathing difficulties
- If you stop breathing for short periods of time when you sleep (called 'sleep apnea syndrome')
- If you have severe liver problems
- In women during breastfeeding
- If you have a history of drug or alcohol abuse
- If you are taking any medicine or products that are not medicines that contain cannabidiol, as it may increase the adverse effects of clobazam.
Clobazam should not be given to children from 1 month to 2 years, except in exceptional situations when antiepileptic treatment is essential.
Tell your doctor if you have kidney failure so that they can monitor you. Your doctor will decide if it is necessary to reduce the dose of Silocalm oral suspension.
If you are an elderly person, your doctor may reduce the dose.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take Silocalm oral suspension.
Change from tablet to oral suspension
Special caution is required when changing from a tablet to an oral suspension, as the doses are not identical. You may experience breathing problems or drowsiness when changing from a clobazam tablet to Silocalm oral suspension.
You may also experience an increase in the frequency of epileptic seizures or the appearance of new types of epilepsy with Silocalm oral suspension. Consult your doctor if you notice these symptoms.
Alcohol
Do not consume alcohol during treatment with clobazam, as there is a greater risk of experiencing adverse effects.
Amnesia (memory loss)
You may observe memory loss during treatment with Silocalm oral suspension when administered within the usual dosage range. This reaction occurs only with high doses.
Muscle weakness
Silocalm oral suspension may cause muscle weakness. Inform your doctor if you observe a lack of coordination (called 'ataxia espinocerebelosa'). In cases of severe muscle weakness (myasthenia gravis), clobazam is not recommended.
Dependence, tolerance, and withdrawal
The use of Silocalm oral suspension may lead to dependence after taking the medicine for a long time or at high doses, especially in patients with a history of drug and alcohol abuse. This means that you may feel the need for continued treatment with Silocalm oral suspension to feel well (known as psychological dependence). Therefore, you should take the medicine for the shortest possible time.
If you stop taking Silocalm oral suspension abruptly, you may experience a worsening of the symptoms that originally led to treatment, as well as mood changes, anxiety, sleep disorders, headache, increased sleep, tension, confusion, excitability, hallucinations, muscle pain, numbness or tingling in the limbs, sweating, tremors, nausea, sensitivity to light, increased sensitivity to sound, or restlessness. This is known as withdrawal syndrome and can be avoided by gradually reducing the dose. Consult your doctor if you are concerned about dependence and withdrawal.
If you take Silocalm oral suspension for a long time to treat epilepsy, you may become accustomed to the medicine, which means that the medicine becomes less effective over time. If you notice that Silocalm oral suspension is no longer helping you control your symptoms, consult your doctor, who may recommend that you take a break from the medicine.
Respiratory depression
Silocalm oral suspension may cause respiratory depression, especially when administered at high doses. Inform your doctor if you have respiratory failure. Your doctor will decide if it is necessary to reduce the dose. In cases of severe breathing difficulties, clobazam should not be administered.
Kidney and liver failure
Inform your doctor if you have kidney or liver failure so that they can monitor you. Your doctor will decide if it is necessary to reduce the dose of Silocalm oral suspension.
Elderly patients
During treatment with clobazam, adverse reactions such as drowsiness, dizziness, muscle weakness, and increased risk of falls that can result in serious injuries are more frequent in patients over 65 years of age than in younger patients. If you are elderly, your doctor may prescribe a lower dose and monitor your response to treatment. Please follow your doctor's instructions carefully.
Serious skin problems
Clobazam may cause serious skin reactions. You should inform your doctor if you experience a skin reaction, unless it is clear that it is not related to the medicine.
Depression and suicidal thoughts
Some patients have experienced suicidal thoughts while taking medicines that contain clobazam, especially if they are already in a depressive state. If you are depressed, have irrational fears and obsessions, have started thinking about suicide or self-harm, inform your doctor immediately.
Psychotic and "paradoxical" reactions
It is known that the use of clobazam can cause restlessness, agitation, irritability, aggression, delirium, rage, nightmares, hallucinations, delusional ideas (psychosis), inappropriate behavior, and other adverse effects on behavior. If this happens, you should stop taking Silocalm oral suspension and contact your doctor. These reactions are more frequent in children and elderly patients.
Slow metabolism
It is possible that in some patients, the liver does not break down medicines properly. In these patients, the medicine may remain in the body for longer, causing adverse effects. Inform your doctor if you know that you metabolize certain medicines slowly.
Children from 1 month to 2 years:
Silocalm oral suspension should not be administered to children under 2 years of age, except if the doctor decides that it is essential.
If Silocalm oral suspension is taken with opioids, it may cause drowsiness, breathing difficulties, coma, and put your life at risk. It should only be considered for use with opioids when other treatment options are inadequate. Inform your doctor of all opioid medicines you are taking and follow your doctor's recommendations regarding the dose strictly.
Taking Silocalm oral suspension with other medicines
Inform your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
At high doses of clobazam, the simultaneous use of more than one medicine may increase or decrease its effect. These medicines include:
- Medicines for treating epilepsy (phenytoin, carbamazepine, valproic acid, stiripentol)
- Medicines for treating depression (trazodone, selective serotonin reuptake inhibitors 'SSRIs' (fluoxetine or citalopram), tricyclic antidepressants (amitriptyline or nortriptyline), or monoamine oxidase inhibitors 'MAOIs' (phenelzine or moclobemide))
- Medicines for treating serious mental illnesses called 'neuroleptics' (such as chlorpromazine, haloperidol, and clozapine)
- Pain relievers (medicines containing codeine, dihydrocodeine, or morphine)
- Anxiolytics (zolpidem)
- Tranquilizers (diazepam, temazepam, or lorazepam)
- Muscle relaxants (baclofen)
- Antihistamines that make you feel drowsy (chlorphenamine, promethazine, or diphenhydramine)
- Lithium: for treating a mental illness called 'bipolar disorder' (mood swings between abnormal excitement and depression)
- Cimetidine (for treating stomach acidity and ulcers)
- The antibiotic erythromycin
- Omeprazole: for treating symptoms of acid reflux such as heartburn and acid regurgitation
- Ticlopidine: an antiplatelet medicine used in patients with a higher risk of suffering from embolism
- Fluconazole (for treating fungal infections)
- Fluvoxamine, paroxetine (for treating depression)
- Dextromethorphan (for relieving dry, irritating coughs)
- Nebivolol (for treating high blood pressure)
- Pimozide (for treating mental disorders)
- Products containing cannabidiol (medicines or non-medicinal products)
The concomitant use of clobazam and opioids (powerful pain relievers, substitution therapy medicines, and some cough medicines) increases the risk of drowsiness, breathing difficulties (respiratory depression), coma, and can put your life at risk. For this reason, concomitant use should only be considered when other treatment options are not possible.
If your doctor still prescribes Silocalm with opioids, you should limit the dose and duration of concomitant treatment.
Inform your doctor of all opioid medicines you are taking and follow your doctor's recommendations regarding the dose strictly. It may be useful to inform friends or family members so that they can recognize the signs and symptoms mentioned above. If you experience these symptoms, contact your doctor.
If you have any doubts about whether what you have read affects you, consult your doctor or pharmacist before using this medicine.
Anesthesia
If you are going to undergo anesthesia, inform your doctor or anesthesiologist before taking Silocalm oral suspension so that they can change the amount of anesthesia or muscle relaxants they will administer.
Taking Silocalm oral suspension with food, drinks, and alcohol
During treatment with Silocalm oral suspension, avoid consuming alcoholic beverages. The effect of alcohol may alter the therapeutic results of Silocalm oral suspension.
Pregnancy, breastfeeding, and fertility
Pregnancy
This medicine should not be used during pregnancy or in women of childbearing potential who do not use contraceptive methods.
If you discover that you are pregnant or plan to become pregnant, consult your doctor immediately to re-evaluate the need for treatment. Do not stop treatment with Silocalm without consulting your doctor.
A large amount of data has not shown evidence of malformations associated with the use of benzodiazepines. However, some studies have shown a risk of cleft lip and palate in newborns that is potentially higher compared to the general population.
Cleft lip and palate (sometimes called "hare lip") is a congenital malformation caused by incomplete fusion of the palate and upper lip.
There may be cases of reduced fetal movement and variability of fetal heart rate after taking clobazam during the second and/or third trimester of pregnancy.
If Silocalm is taken at the end of pregnancy or during childbirth, your baby may show drowsiness (sedation), muscle weakness (hypotonia or neonatal hypotonic syndrome), a drop in body temperature (hypothermia), difficulty feeding (lactation problems, resulting in poor weight gain), and breathing problems (respiratory depression, sometimes severe).
If you take this medicine regularly in the late stage of pregnancy, your baby may experience withdrawal symptoms such as agitation or tremors. In such cases, the newborn should be closely monitored during the postnatal period.
Breastfeeding
Since clobazam, the active ingredient of Silocalm oral suspension, is excreted in breast milk, this oral suspension should not be used during breastfeeding .
Driving and using machines
The influence of clobazam on the ability to drive and use machines is significant.
Silocalm may alter your ability to drive or operate machinery, as it may cause drowsiness, reduce your attention, or reduce your reaction time. You may also experience double vision. The occurrence of these effects is more likely at the start of treatment or when the dose is increased. Do not drive or use machines if you experience any of these effects.
Consult your doctor if you can drive safely while taking this medicine.
Silocalm oral suspension containssorbitol, methylparaben sodium, propylparaben sodium, sodium, and propylene glycol:
- This medicine contains 250 mg of sorbitol per ml of suspension. Sorbitol is a source of fructose. If your doctor has told you that you (or your child) have an intolerance to certain sugars, or you have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disorder in which the patient cannot break down fructose, consult your doctor before taking this medicine. Sorbitol may cause gastrointestinal discomfort and a mild laxative effect.
- This medicine contains 2.06 mg of methylparaben sodium and 0.224 mg of propylparaben sodium per ml of suspension. It may cause allergic reactions (possibly delayed) and, exceptionally, bronchospasm.
- This medicine contains 3.33 mg of sodium (main component of table salt/cooking salt) per ml of suspension. The maximum dose is equivalent to 10% of the maximum recommended daily sodium intake for an adult.
- This medicine contains 4.825 mg of propylene glycol per ml of suspension. If your child is under 5 years old, consult your doctor or pharmacist before giving them this medicine, especially if they are using other medicines that contain propylene glycol or alcohol. If you are pregnant or breastfeeding, do not take it unless your doctor recommends it. Your doctor may perform additional checks while you are taking this medicine. If you have liver or kidney disease, do not take this medicine unless your doctor recommends it. Your doctor may perform additional checks while you are taking this medicine.
If you have any questions about whether this medicine is suitable for you, consult your doctor, pharmacist, or nurse.
3. How to take Silocalm
Follow exactly the administration instructions of this medication as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
In most cases, Silocalm oral suspension is administered for 2 to 4 weeks. After each 4 weeks, your doctor will decide if you should continue taking this medication. Consult your doctor or pharmacist if you have doubts.
When taking Silocalm oral suspension, you should not switch to another medication containing clobazam unless under the supervision of your doctor.
When low doses are needed, the 1mg/ml presentation is the most suitable. When high doses are needed, the 2mg/ml presentation is the most suitable.
The recommended dose is
Adults and adolescents
- The initial dose is 5 to 15 mg per day, gradually increasing as needed.
- Your doctor may increase the dose up to a maximum of 60 mg per day.
- Your doctor may reduce the dose based on your response to treatment.
Use in children (between 2 and 16 years)
- The initial dose is 5 mg per day for children from 6 years of age or 0.1 mg/kg/day for younger patients, gradually increasing as needed every 7 days.
- The usual maintenance dose is between 0.3 and 1 mg/kg per day, administered in two separate doses or in a single dose at night.
- The doctor will adjust the dose according to the child's needs.
Generally, the use of clobazam is not suitable for children under 2 years of age. However, it may be administered under specialized medical supervision.
In patients with liver or kidney disease and elderly patients, low initial doses are required, with gradual increases and under careful observation by their doctor (see section "Warnings and precautions").
Method of administration
Sediments may form during storage. Shake the bottle well before using it.
Your doctor, nurse, or pharmacist will indicate how to administer this medication. A 5 ml dosing syringe, a syringe adapter, and a 30ml dosing cup are provided with the medication packaging.

5 ml syringe: each 1 ml graduation line on the syringe is equivalent to 1 mg of Silocalm 1 mg/ml oral suspension. The smaller graduation lines are 0.2 ml or 0.2 mg of Silocalm 1mg/ml oral suspension.

30 ml dosing cup: each 5 ml graduation line on the cup is equivalent to 5 mg of Silocalm 1mg/ml oral suspension.
Instructions for using the dosing syringe are on the back. If you have any questions about the dose you should take or how to use the syringe, ask your pharmacist.
Instructions for use:
Open the bottle, press down the cap, and turn it counterclockwise (figure 1)
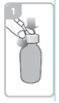
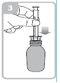
Holding the bottle, remove the plastic syringe adapter from the packaging and insert the adapter into the bottle neck (figure 2). Make sure it is securely fixed.

Take the syringe and place it in the adapter opening (figure 3). Place the bottle upside down.
Fill the syringe with a small amount of suspension by pulling the plunger down (figure 4a), and then push the plunger up to eliminate any possible bubbles (figure 4b). Pull the plunger down to the graduation mark that corresponds to the amount in milliliters (ml) prescribed by your doctor (figure 4C).
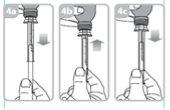
Put the bottle in a vertical position.
Remove the syringe from the adapter (figure 5)

Administer the entire contents of the syringe into the mouth by pushing the plunger to the bottom of the syringe (figure 6) and check that the medication has been swallowed.
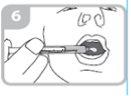
Remove the adapter from the bottle and close the bottle with the plastic cap.
Wash the adapter and syringe with warm water, dry them with a clean paper towel, and put them back in the packaging with your medication.
If you take moreSilocalmoral suspension than you should
If you take more Silocalm oral suspension than you should, consult your doctor or go to the nearest hospital immediately. Take the medication packaging with you . Do notdrive because you may start to feel drowsy.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to takeSilocalmoral suspension
If you have forgotten a dose, take it as soon as you realize it, unless it is close to the time of the next dose, and then continue as before. Do nottake a double dose to make up for forgotten doses.
If you interrupt treatment withSilocalmoral suspension
Do notinterrupt the medication, but rather graduallyreduce the dose according to the doctor's instructions before interrupting the medication completely. If you interrupt its administration abruptly, you may experience unpleasant adverse effectssuch as stress (anxiety), confusion, or depression. You may also lose your appetite and have difficulty sleeping (see Section 2 'Dependence, tolerance, and withdrawal').
If you have any other doubts about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
Severe adverse effects:
Frequent adverse effects (may affect up to 1 person in 10):
- Feeling of irritability or restlessness.
Uncommon adverse effects (may affect up to 1 person in 100):
- Poor memory while taking Silocalm oral suspension (amnesia) or unusual behavior.
- Nightmares.
- Feeling of anxiety.
- Believing things that are not real (delusions).
- Greater likelihood of stumbling or falling, especially in elderly patients.
Unknown frequency (cannot be estimated from available data)
- Sleep problems that worsen after taking this medication.
- Noticing things that are not there (hallucinations).
- Being less aware of your surroundings, especially in the elderly.
- Feeling suicidal.
- Blisters or bleeding of the skin around the lips, eyes, mouth, nose, and genitals. Also, flu-like symptoms and fever. This may be something called 'Stevens-Johnson Syndrome'.
- A skin rash with severe blisters where the skin layers can peel off, leaving large areas of exposed skin on the body. Also, a feeling of general discomfort, fever, chills, and muscle pain. This is something called 'Toxic Epidermal Necrolysis'.
If you experience any of the above-mentioned adverse effects, your doctor will decide if you should interrupt treatment.
Tell your doctor or pharmacist if any of the following adverse effects worsen or last more than a few days, or if you notice any adverse effect not mentioned in this leaflet.
Very frequent adverse effects (may affect more than 1 person in 10):
- Difficulty staying awake or alert.
Frequent adverse effects (may affect up to 1 person in 10):
- Feeling of drowsiness or dizziness.
- Feeling of agitation or aggression.
- Depression.
- Headache.
- Short attention span.
- Difficulty speaking.
- Tremors in the fingers.
- Problems walking or other movement problems.
- Dry mouth, constipation.
- Lack of appetite, nausea.
Uncommon adverse effects (may affect up to 1 person in 100):
- Loss of sexual desire in long-term treatments or with high doses, reversible in nature.
- Memory difficulties, confusion.
- Double vision.
- Skin rash.
• Weight gain.
Unknown frequency (cannot be estimated from available data):
- Dependence ("physical or psychological") on Silocalm oral suspension (especially in long-term treatment).
- Feeling of not being in touch with reality and being unable to think or judge clearly (psychosis).
- Feeling of anger.
- Changes in the way of walking.
- Breathing problems.
- Sensitivity to sunlight.
- Skin rash with swelling and itching (urticaria).
- Muscle spasms or muscle weakness.
- Slower than normal reaction to things.
- Uncontrolled rapid eye movement.
- Learning problems.
- Abnormally low body temperature.
If you take this medication for a prolonged period, you are more likely to experience the following adverse effects:
anxiety, confusion, depression, loss of appetite, and difficulty sleeping.
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect not mentioned in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Silocalm oral suspension
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the bottle label and packaging after CAD. The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C. Once opened, use before 28 days.
Medications should not be thrown down the drain or into the trash. Deposit the packaging and medications you no longer need at the SIGRE point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Packaging contents and additional information
Silocalm oral suspension composition
- The active principle (the component that allows the oral solution to work) is clobazam. Each ml contains 1 mg of clobazam.
- The other components are sorbitol (E420), xanthan gum (E415), acesulfame potassium (E950), raspberry flavor (contains propylene glycol (E 1520), sodium propyl parahydroxybenzoate (E217), sodium methyl parahydroxybenzoate (E219), disodium hydrogen phosphate dihydrate, sodium dihydrogen phosphate dihydrate, and purified water.
Product appearance and packaging contents
Silocalm oral suspension is a viscous suspension with a white color and a raspberry flavor, supplied in an amber glass bottle.
Sediments may form during the storage of this product. Shake the bottle vigorously before using it.
Package sizes: 100 ml, 150 ml, and 250 ml.
A 30 ml dosing cup and a 5 ml syringe are provided with the packaging.
Not all package sizes may be marketed.
Marketing authorization holder
ETHYPHARM,
194 Bureaux de la Colline Batiment D 92213
Saint-Cloud Cedex, France
Tel: +33(0)141121720
Manufacturer
ETHYPHARM,
Chemin de la Poudriere, GRAND QUEVILLY,76120, France
Local representative:
Altan Pharmaceuticals, S.A.
C/ Cólquide, Nº 6, Portal 2, 1ª Planta, Oficina F. Edificio Prisma, Las Rozas,
28230 Madrid - Spain
This medication is authorized in the member states of the European Economic Area with the following names:
Germany: Epaclob® 1 mg/ml Suspension zum Einnehmen
Denmark: Silocalm®
Spain: Silocalm® 1 mg/ml oral suspension
Italy: Epaclob® 1 mg/ml
Ireland: Epaclob® 1 mg/ml oral suspension
Date of the last revision of this leaflet: 08/2023
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SILOCALM 1 mg/ml ORAL SUSPENSIONDosage form: TABLET, 10 mg clobazamActive substance: clobazamManufacturer: Atnahs Pharma Netherlands Bv.Prescription requiredDosage form: TABLET, 20 mg clobazamActive substance: clobazamManufacturer: Atnahs Pharma Netherlands Bv.Prescription required
Online doctors for SILOCALM 1 mg/ml ORAL SUSPENSION
Discuss questions about SILOCALM 1 mg/ml ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











