
SEPTOCIPRO OTIC 1 mg OTIC DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS


How to use SEPTOCIPRO OTIC 1 mg OTIC DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SEPTOCIPRO OTIC 1mg ear drops in solution in single-dose container
Ciprofloxacin
Read the entire package leaflet carefully before starting to use this medication.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you consider that any of the side effects you are suffering from is serious or if you notice any side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Contents of the Package Leaflet
- What SEPTOCIPRO OTIC is and what it is used for
- What you need to know before starting to use SEPTOCIPRO OTIC
- How to use SEPTOCIPRO OTIC
- Possible side effects
- Storage of SEPTOCIPRO OTIC
- Contents of the pack and further information
1. What SEPTOCIPRO OTIC is and what it is used for
The active substance of the medication is ciprofloxacin, an antibiotic that belongs to a group of antibacterial agents known as quinolones. Ciprofloxacin prevents the growth of certain microorganisms that cause infections.
Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential that you follow the instructions regarding the dose, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medications down the drain or in the trash.
SEPTOCIPRO OTIC is used for the topical treatment in adults and children aged 2 years or older of the following bacterial ear infections: chronic suppurative otitis media and acute otitis externa.
2. What you need to know before starting to use SEPTOCIPRO OTIC
Do not use SEPTOCIPRO OTIC
If you are allergic to ciprofloxacin, other quinolones, or any of the other components of this medication (see section 6).
Be cautious with SEPTOCIPRO OTIC
- If you experience an allergic reaction to SEPTOCIPRO OTIC, stop the treatment and inform your doctor. Allergic reactions are rare and severe reactions are even rarer (see section 4 of this package leaflet).
- Prolonged treatment with this medication may cause the proliferation of resistant microorganisms. If you do not experience an improvement in your otitis, inform your doctor.
- If you have a perforated eardrum (the membrane that separates the outer ear from the middle ear), the medication may penetrate the oral cavity.
- If your skin becomes more sensitive to sunlight or ultraviolet (UV) light while taking ciprofloxacin, avoid exposure to intense sunlight or artificial UV light, such as that from sunbeds.
Use of other medications
Inform your doctor or pharmacist if you are using or have recently used or may need to use any other medication. SEPTOCIPRO OTIC should not be administered with other medications via the otic route (through the ear).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
It is recommended to avoid using SEPTOCIPRO OTIC during pregnancy. Inform your doctor if you plan to become pregnant.
Do not use SEPTOCIPRO OTIC during breastfeeding, as ciprofloxacin may pass into breast milk and may be harmful to your child.
Driving and using machines
Among the side effects described with this medication are dizziness (a feeling of dizziness that may be accompanied by discomfort, nausea, and vomiting, etc.) and headache (see section 4). Therefore, make sure you know how you react to SEPTOCIPRO OTIC before driving a vehicle or using machines. In case of doubt, consult your doctor.
3. How to use SEPTOCIPRO OTIC
For children over 2 years, adolescents, and adults:
Follow the administration instructions of SEPTOCIPRO OTIC exactly as indicated by your doctor. Your doctor will indicate precisely how much SEPTOCIPRO OTIC you should receive, as well as how often and for how long, depending on the type of infections you have and their severity. In case of doubt, consult your doctor or pharmacist again.
SEPTOCIPRO OTIC should be administered only via the otic route (through the ear). The usual dose is as follows:
- Acute otitis externa: 1 mg (one single-dose container of 0.5 ml) every 12 hours for 7 days.
- Chronic suppurative otitis media: 1 mg (one single-dose container of 0.5 ml) every 12 hours for 15 days.
Elderly patients
For patients over 65 years, the dose is the same. If you are unsure, consult your doctor or pharmacist.
To administer correctly, follow these instructions:
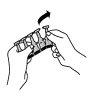 1. Remove a unit from the strip of containers.
1. Remove a unit from the strip of containers.
2. Check that the container is not damaged.
3. Hold the container between the index and thumb fingers of one hand.
With the index and thumb fingers of the other hand, perform a slight lever action on the winged piece to open it. Do not twist to break.
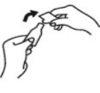
4. Remove the winged piece.

5. To apply the otic solution correctly, you must remain lying down, with the ear to be treated facing upwards.
6. To facilitate the penetration of the otic solution into the ear, you must hold the ear by the upper part and pull it back. In this position, empty the contents of the container into the ear, pressing the single-dose container several times. The amount of solution contained in the single-dose container guarantees the application of the correct dose (0.5 ml), even if a small amount remains inside after emptying.

7. Once the otic solution has been applied, you must press the prominence of the ear located in front of the external auditory canal (called the tragus) several times to facilitate the penetration of the otic solution into the ear.

8. The otic solution should remain in the ear for about 5 minutes.
9. Afterwards, you should sit up and tilt your head to the side of the treated ear so that the excess otic solution drains out.

It is not recommended to "plug" the ear with a cotton ball or similar object; this may prolong the duration of the infection.
Use in children
There is no information on the use of SEPTOCIPRO OTIC in children under 2 years of age; therefore, this medication is not recommended for this age group.
If you use more SEPTOCIPRO OTIC than you should
In case of overdose or accidental ingestion, inform your doctor or pharmacist or call the Toxicological Information Service, phone: 91 562 04 20, indicating the medication and quantity ingested.
If you forget to use SEPTOCIPRO OTIC
Do not use a double dose to make up for the forgotten dose.
If you interrupt treatment with SEPTOCIPRO OTIC
It is essential that you complete the treatment cycle, even if you start to feel better after a few days. If you stop using this medication too soon, it is possible that the infection has not been completely cured, and the symptoms may return or worsen. Resistance to the antibiotic may also develop.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, SEPTOCIPRO OTIC can cause side effects, although not everyone will experience them.
If you experience a severe allergic reaction and notice any of the following problems, stop using ciprofloxacin immediately and inform your doctor: swelling of the hands, feet, ankles, face, lips, mouth, or throat that may cause difficulty swallowing or breathing, skin rash or hives, large blisters filled with fluid, pain, and ulcers.
Common side effects (affecting 1 to 10 in every 100 patients): proliferation of microorganisms resistant to this medication in the ear.
Uncommon side effects (affecting 1 to 10 in every 1,000 patients): dizziness and headache. Localized itching, pain, and a burning or stinging sensation at the site of administration may also occur.
Rare side effects (affecting 1 to 10 in every 10,000 patients): allergic reactions that can affect the entire body.
If you consider that any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist or nurse, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of SEPTOCIPRO OTIC
Keep this medication out of the sight and reach of children.
Store the single-dose containers in the outer packaging to protect them from light.
Do not use SEPTOCIPRO OTIC after the expiration date stated on the container after "EXP". The expiration date is the last day of the month indicated.
Medications should not be disposed of via wastewater or household waste. Deposit the containers and medications you no longer need at the pharmacy's collection point. If you have any doubts, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Further information
Composition of SEPTOCIPRO OTIC
- The active substance of SEPTOCIPRO OTIC is ciprofloxacin. Each single-dose container of 0.5 ml contains 1 mg of ciprofloxacin (as hydrochloride).
- The other components are glycerol (E422), polysorbate 20, sodium acetate, glacial acetic acid, methylcellulose (E461), sodium hydroxide or hydrochloric acid, and water for injectable preparations.
Product characteristics and container contents
SEPTOCIPRO OTIC is supplied in single-dose containers of 0.5 ml of sterile solution. Each carton contains 20 single-dose containers.
Marketing authorization holder
Neuraxpharm Spain, S.L.U.
Avda. Barcelona, 69
08970 Sant Joan Despí
Barcelona – Spain
Manufacturer
Neuraxpharm Pharmaceuticals, S.L.
Avda. Barcelona, 69
08970 Sant Joan Despí (Barcelona)
Spain
Date of the last revision of this package leaflet: July 2013
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.es/
- Country of registration
- Average pharmacy price5.01 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SEPTOCIPRO OTIC 1 mg OTIC DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: OTIC SOLUTION, 3 mg/mlActive substance: ciprofloxacinManufacturer: Zambon S.A.U.Prescription requiredDosage form: OTIC SOLUTION, 1.2 mg of ciprofloxacin in 0.4 mlActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: OTIC SOLUTION, 3 mgActive substance: ciprofloxacinManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for SEPTOCIPRO OTIC 1 mg OTIC DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about SEPTOCIPRO OTIC 1 mg OTIC DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








