
SENSEDOL 0.075% CREAM


How to use SENSEDOL 0.075% CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Sensedol 0.075% Cream
Oleoresin of Capsicum annuumL. (equivalent to 0.075% capsaicin)
Read this package leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Sensedol and what is it used for
- What you need to know before using Sensedol
- How to use Sensedol
- Possible side effects
- Storage of Sensedol
- Contents of the pack and further information
1. What is Sensedol and what is it used for
Sensedol contains oleoresin of Capsicum annuumL., which is a local anesthetic active ingredient.
It is indicated for the relief of moderate to severe pain in painful diabetic neuropathy that interferes with daily activities and has not responded to other treatments in adults.
2. What you need to know before using Sensedol
Do not use Sensedol
If you are allergic to oleoresin of Capsicum annuumL. or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Sensedol.
This treatment should be initiated and supervised by the specialist who treats the diabetic patient. This medicine is for external use only. Do not apply to irritated skin or wounds. The product is highly irritating. Avoid contact with eyes and mucous membranes. To do this, it is recommended to always wash your hands well with cold water and soap immediately after each application, and unless indicated by the doctor, application of the cream near the eyes or on mucous membranes (e.g., the mouth) should be avoided. Contact with the eyes or other mucous membranes can cause a burning sensation. If this occurs, the area should be rinsed with plenty of cold water.
When the treated area is the hands, patients should not wash them for at least 30 minutes after application. During this time, accidental contact with sensitive areas should be monitored.
If the pain persists or worsens after the first 2 weeks of treatment, or disappears and returns after a few days, or excessive irritation appears, treatment should be discontinued and the doctor consulted.
Do not apply heat or tight bandages to the area. Do not use for prolonged periods or on large areas.
Children and adolescents
Not recommended.
Other medicines and Sensedol
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Possible interactions of this medicine with other topical medicines are not known.
As this is a topical product, interactions with other systemic medicines are not expected.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
If you are pregnant, your doctor will decide whether to start this treatment.
Similarly, if you are breastfeeding, your doctor will decide whether to start treatment with this medicine.
Driving and using machines
Using this medicine does not affect your ability to drive or use machines.
Sensedol contains cetyl alcohol, methylparaben sodium (E-219), propylparaben sodium (E-217), and propylene glycol (E-1520)
This medicine may cause local skin reactions (such as contact dermatitis) because it contains cetyl alcohol.
This medicine may cause allergic reactions (possibly delayed) because it contains methylparaben sodium (E-219) and propylparaben sodium (E-217).
This medicine contains 50 mg of propylene glycol per gram of cream.
3. How to use Sensedol
Follow exactly the administration instructions of this medicine as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Use in adults and elderly patients
The recommended dose is three or four applications per day for 8 weeks, after which the doctor will determine whether to suspend or continue treatment.
Use in children and adolescents
Not recommended.
Sensedol is a medicine for external use only. It should be applied to the painful areas of the skin.
Instructions for correct administration
The tube must be pierced before its first application. Pierce the tube and apply following these instructions:
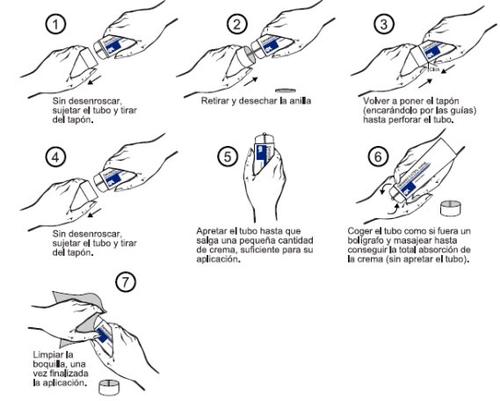
Follow these instructions unless your doctor has given you different instructions.
Apply the minimum amount of cream necessary to cover the affected skin area.
Spread the cream with a gentle massage until it is fully absorbed, avoiding any remaining residue, as indicated in graph 6 of section 3.
It is important to make daily applications.
The duration of treatment will be 8 weeks, after which the doctor will determine whether to suspend or continue treatment.
Your doctor will indicate the duration of your treatment with Sensedol.
If you use more Sensedol than you should
Acute intoxication is practically impossible with the proper use of this medicine.
In case of overdose or accidental ingestion, or contact with the eyes, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Sensedol
Do not make a double application to compensate for forgotten doses.
Continue with the treatment as recommended.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
During the first few days of treatment, a burning or itching sensation may appear in the application area in approximately 50% of cases. This known reaction is a consequence of the pharmacological action of capsaicin, releasing substance P from peripheral nerve endings and accumulating in the synapse, and usually disappears or decreases over time as treatment continues at the recommended dose, without the need to interrupt it. Its duration and intensity are variable but may be prolonged if Sensedol is applied less than 3 or 4 times a day. Hot water, excessive sweating, or occlusion may intensify this sensation. Other possible side effects at the skin level may be irritative erythema and dryness of the skin at the application site.
During treatment, estornudos, lagrimeo, or tos (less than 2%) may also appear, as a consequence of inhaling dry cream residues. Therefore, it is essential to apply the minimum amount of cream necessary and avoid leaving residues on the skin, as well as washing hands with cold water and soap after use.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's (AEMPS) online system: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sensedol
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging. The expiry date is the last day of the month indicated.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Sensedol
- The active ingredient is oleoresin of Capsicum annuumL. Each 100 grams of Sensedol 0.075% cream contains 312-625 mg of oleoresin of Capsicum annuumL., equivalent to 75 mg of capsaicin.
- The other ingredients (excipients) are: stearic acid; cetyl alcohol; oleic acid; isopropyl myristate; glycerol monostearate; polyethylene glycol monostearate; methylparaben sodium (E-219); propylparaben sodium (E-217); propylene glycol (E-1520); purified water.
Appearance and packaging of the product
Sensedol is a white-yellowish cream.
This medicine is available in 30 and 50 gram packs.
Marketing authorization holder and manufacturer
Especialidades Farmacéuticas Centrum, S.A.
C/ Sagitario, 14
03006 Alicante (Spain)
Grupo Asacpharma
Date of the last revision of this package leaflet:March 2019.
Other sources of information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Average pharmacy price12.91 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SENSEDOL 0.075% CREAMDosage form: CREAM, 0.075% Capsaicin (30 and 50 g cream)Active substance: capsaicinManufacturer: Alfasigma Espana S.L.Prescription requiredDosage form: CREAM, 0.075 g capsaicinActive substance: capsaicinManufacturer: Laboratorios Vinas S.A.Prescription requiredDosage form: CREAM, 75 mg Capsaicin / 100 g CreamActive substance: capsaicinManufacturer: Arafarma Group S.A.Prescription required
Online doctors for SENSEDOL 0.075% CREAM
Discuss questions about SENSEDOL 0.075% CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















