
RYSTIGGO 140 mg/ml INJECTABLE SOLUTION

How to use RYSTIGGO 140 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Rystiggo 140 mg/ml Solution for Injection
rozanolixizumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Pack
- What is Rystiggo and what is it used for
- What you need to know before you use Rystiggo
- How to use Rystiggo
- Possible side effects
- Storing Rystiggo
- Contents of the pack and other information
1. What is Rystiggo and what is it used for
What is Rystiggo
Rystiggo contains the active substance rozanolixizumab. Rozanolixizumab is a monoclonal antibody (a type of protein) designed to recognize and bind to FcRn, a protein that keeps immunoglobulin G (IgG) antibodies in the body for longer.
Rystiggo is used, along with standard treatment, in adults to treat generalized myasthenia gravis (gMG), an autoimmune disease that causes muscle weakness and affects multiple muscle groups in the body. The condition can also cause difficulty breathing, extreme fatigue, and difficulty swallowing. Rystiggo is used in adults with gMG who produce IgG autoantibodies against acetylcholine receptors or muscle-specific kinase.
In generalized myasthenia gravis (gMG), these IgG autoantibodies (proteins from the immune system that attack parts of a person's own body) attack and damage the proteins involved in communication between nerves and muscles, known as acetylcholine receptors or muscle-specific kinase.
By binding to FcRn, Rystiggo reduces the amount of IgG antibodies, including autoantibodies (antibodies against one's own body), thereby helping to improve the symptoms of the disease.
2. What you need to know before you use Rystiggo
Do not use Rystiggo
- If you are allergic to rozanolixizumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting treatment with this medicine if you are in any of the following situations:
Myasthenic crisis
Your doctor may not prescribe this medicine if you are using a respirator, or are likely to do so, due to muscle weakness caused by gMG (myasthenic crisis).
Inflammation of the membranes surrounding the brain and spinal cord (aseptic meningitis)
Aseptic meningitis has been observed in association with this medicine. Seek immediate medical attention if you develop symptoms of aseptic meningitis, such as severe headache, fever, stiff neck, nausea, vomiting, and/or intolerance to bright light.
Infections
This medicine may reduce your natural resistance to infections. Before starting treatment with this medicine or during treatment, tell your doctor if you have any symptoms of infection (feeling hot, fever, chills or shivering, cough, sore throat, or cold sores may be signs of an infection).
Hypersensitivity (allergic reactions)
This medicine contains a protein that can cause reactions such as skin rash, swelling, or itching in some people. You will be monitored for signs of an infusion reaction during treatment and 15 minutes after treatment.
Vaccinations (vaccines)
Tell your doctor if you have received a vaccine in the last 4 weeks or are planning to be vaccinated soon.
Children and adolescents
Do not give this medicine to children under 18 years of age, as the use of Rystiggo has not been studied in this age group.
Other medicines and Rystiggo
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
The use of Rystiggo with other medicines may reduce the effectiveness of those medicines, including therapeutic antibodies (such as rituximab) or subcutaneous or intravenous immunoglobulins. Other medicines, including subcutaneous or intravenous immunoglobulins, or interventions such as plasmapheresis (a process in which the liquid part of the blood, or plasma, is separated from the blood drawn from a person), may affect the effect of Rystiggo. Tell your doctor if you are taking or plan to take any other medicine.
Before being vaccinated, tell your doctor about your treatment with Rystiggo. This medicine may affect the effect of vaccines. Vaccination with live or attenuated vaccines is not recommended during treatment with Rystiggo.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
The effects of this medicine in pregnancy are not known. You should not use this medicine if you are pregnant or think you may be pregnant, unless your doctor has specifically recommended it.
It is not known whether this medicine passes into breast milk. Your doctor will help you decide whether to breastfeed and use Rystiggo.
Driving and using machines
Rystiggo is unlikely to affect your ability to drive or use machines.
Rystiggo contains proline
This medicine contains 29 mg of proline per milliliter of medicine.
Proline may be harmful to patients with hyperprolinemia, a rare genetic disorder in which an excess of the amino acid proline accumulates in the body.
If you have hyperprolinemia, tell your doctor and do not use this medicine unless it has been recommended.
Rystiggo contains polysorbate 80
This medicine contains 0.3 mg of polysorbate 80 per milliliter of medicine. Polysorbates can cause allergic reactions. Tell your doctor if you have any known allergies.
3. How to use Rystiggo
Treatment with Rystiggo will be started and supervised by a specialist doctor with experience in the treatment of neuromuscular or neuroinflammatory disorders.
How much Rystiggo is given and for how long
You will be given Rystiggo in cycles of 1 infusion per week for 6 weeks.
Your doctor will calculate the correct dose for you based on your weight:
The frequency of treatment cycles varies for each patient, and your doctor will consider whether it is appropriate for you to receive a new treatment cycle and, if so, when.
Your doctor will tell you how long you should receive treatment with this medicine.
How Rystiggo is given
Rystiggo will be given to you by a doctor or nurse.
You can also inject Rystiggo yourself. You and your doctor or nurse will decide if, after receiving training from a healthcare professional, you can inject this medicine yourself. Someone else can also give you the injections after receiving training. Do not inject Rystiggo yourself or allow someone else to inject it until you have been taught how to do so.
If you or your caregiver give Rystiggo injections, you must carefully read and follow the Administration Instructions at the end of this leaflet (see "Instructions for use").
You will receive this medicine by infusion under the skin (subcutaneously). It is usually injected into the lower abdomen, below the navel. Injections should not be given in areas where the skin is painful, bruised, red, or hardened.
Administration is performed using an infusion pump at a flow rate of up to 20 ml/h.
It can also be given manually (by slow manual injection, i.e., without an infusion pump) at a speed that is comfortable for you.
If you receive more Rystiggo than you should
If you suspect that you have accidentally received a dose of Rystiggo higher than prescribed, ask your doctor for advice.
If you miss or skip a Rystiggo appointment
If you miss a dose, contact your doctor immediately for advice and to schedule another appointment to receive Rystiggo within the next 4 days. Then, the next dose should be given according to the original administration schedule until the treatment cycle is completed.
If you stop treatment with Rystiggo
Do not stop using this medicine without talking to your doctor first. Stopping or interrupting treatment with Rystiggo may cause the symptoms of generalized myasthenia gravis to return.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects, listed in descending order of frequency, have been observed with Rystiggo:
Very common:may affect more than 1 in 10 people
- Headache (including migraine)
- Diarrhea
- Fever (pyrexia)
Common:may affect up to 1 in 10 people
- Rapid swelling under the skin in areas such as the face, throat, arms, and legs (angioedema)
- Joint pain (arthralgia)
- Skin rash, sometimes with red bumps (papular rash)
- Reaction at the injection site, such as skin rash at the injection site, redness of the skin (erythema), swelling, discomfort, and pain at the infusion site
- Nose and throat infections
- Nausea
- Vomiting
Frequency not known(cannot be estimated from the available data):
- non-infectious reversible inflammation of the membranes surrounding the brain and spinal cord (aseptic meningitis):
- headache
- fever
- stiff neck
- nausea
- vomiting
- and/or intolerance to bright light
- viral infections (including herpes zoster and herpes labialis)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Rystiggo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the vial and the outer packaging after the expiry date (EXP). The expiry date refers to the last day of the month shown.
Store in a refrigerator (2°C to 8°C).
Do not freeze.
Keep the vial in the original packaging to protect it from light.
Each vial of solution for injection is for single use only (single dose). Any unused product or material should be disposed of in accordance with local regulations.
Do not use this medicine if you notice that the liquid is cloudy, contains foreign particles, or has changed color.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Rystiggo Composition
- The active ingredientis rozanolixizumab. Each ml of solution contains 140 mg of rozanolixizumab. Each 2 ml vial contains 280 mg of rozanolixizumab. Each 3 ml vial contains 420 mg of rozanolixizumab. Each 4 ml vial contains 560 mg of rozanolixizumab. Each 6 ml vial contains 840 mg of rozanolixizumab.
- The other componentsare: histidine, histidine hydrochloride monohydrate, proline, polysorbate 80, and water for injectable preparations. See section 2 Rystiggo contains proline and Rystiggo contains polysorbate 80.
Product Appearance and Container Contents
Rystiggo is an injectable solution. Each container contains 1 vial of 2 ml, 3 ml, 4 ml, or 6 ml of injectable solution.
Only some vials may be marketed.
The solution is colorless to pale yellowish-brown, transparent to slightly opalescent (pearl white).
Administration materials must be acquired separately.
Marketing Authorization Holder
UCB Pharma S.A., Allée de la Recherche 60, B-1070 Brussels, Belgium
Manufacturer
UCB Pharma S.A., Chemin du Foriest, B-1420 Braine-l’Alleud, Belgium.
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien UCB Pharma S.A./NV Tel: + 32 / (0)2 559 92 00 | Lietuva UAB Medfiles Tel: + 370 5 246 16 40 |
| Luxembourg/Luxemburg UCB Pharma S.A./NV Tél/Tel: + 32 / (0)2 559 92 00 (Belgique/Belgien) |
Ceská republika UCB s.r.o. Tel: + 420 221 773 411 | Magyarország UCB Magyarország Kft. Tel.: + 36-(1) 391 0060 |
Danmark UCB Nordic A/S Tlf.: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: + 49 /(0) 2173 48 4848 | Nederland UCB Pharma B.V. Tel: + 31 / (0)76-573 11 40 |
Eesti OÜ Medfiles Tel: + 372 730 5415 | Norge UCB Nordic A/S Tlf: + 47 / 67 16 5880 |
Ελλάδα UCB Α.Ε. Τηλ: + 30 / 2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Polska UCB Pharma Sp. z o.o. / VEDIM Sp. z o.o. Tel.: + 48 22 696 99 20 |
France UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 21 302 5300 |
Hrvatska Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | România UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Slovenija Medis, d.o.o. Tel: + 386 1 589 69 00 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: + 358 9 2514 4221 |
Κύπρος Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 056300 | Sverige UCB Nordic A/S Tel: + 46 / (0) 40 294 900 |
Latvija Medfiles SIA Tel: + 371 67 370 250 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
-------------------------------------------------------------------------------------------------------------
INSTRUCTIONS FOR USE
Rystiggo (rozanolixizumab)Rystiggo 140 mg/ml solution for subcutaneous injection
Single-use vial
Read all of these instructions before starting to use Rystiggo. A doctor or nurse will show you how to administer Rystiggo before you use it for the first time. It is also possible for another person to administer the infusions after receiving the necessary training. Do not administer Rystiggo or have it administered to another person until you have been taught how to do so. This information does not replace consultation with your doctor about your disease or treatment.
If you are going to use an infusion pump (also called a syringe pump) to administer Rystiggo, read the instructions for setting up the pump provided by your doctor or nurse.
 ! Important information you should know before administering Rystiggo or having it administered to another person
! Important information you should know before administering Rystiggo or having it administered to another person
- For subcutaneous use only (under the skin).
- Use each vial only once.
- Check your dose (you may need more than 1 vial to prepare the prescribed dose).
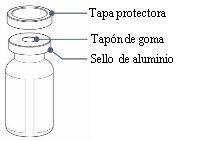 Before using Rystiggo, check that the dose indicated on the vial label matches the dose that has been prescribed for you. Do notuse it if the dose is not the same as the prescribed dose. Contact your doctor or nurse for instructions on what to do next.
Before using Rystiggo, check that the dose indicated on the vial label matches the dose that has been prescribed for you. Do notuse it if the dose is not the same as the prescribed dose. Contact your doctor or nurse for instructions on what to do next.
- Do notuse the vial if the liquid contains visible particles. The medication should be colorless to pale yellowish-brown and transparent to slightly opalescent (pearl white).
- Do notshake the vial.
- Do notuse the vial if the protective cap is missing or defective. If any of the vials are broken or do not have a cap, notify and return them to the pharmacy.
- If you are going to use a non-programmable pump, follow the manufacturer's instructions for use and your nurse's instructions for filling the infusion tube and setting the dose.
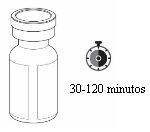 Rystiggo Storage
Rystiggo Storage
- Store in a refrigerator (between 2°C and 8°C).
- Do notfreeze.
- Keep Rystiggo in the original carton to protect it from light.
- Remove the carton from the refrigerator before infusion. For a more comfortable infusion, let the vial reach room temperature before using the medication. This may take between 30 and 120 minutes. Do not heat it in any other way.
! Keep this medication out of the sight and reach of children.
Contents of the Carton
- 1 vial of Rystiggo (2 ml, 3 ml, 4 ml, or 6 ml depending on the dose prescribed for you).
- Rystiggo package leaflet with Instructions for Use.
Step-by-Step Instructions
- Prepare
Step1:Gather all the materials you will need on a clean and flat work surface:
- Includedin the Rystiggo carton:
- Rystiggo vial.
- Rystiggo package leaflet.
! Check your dose (you may need more than 1 vial to prepare the prescribed dose).
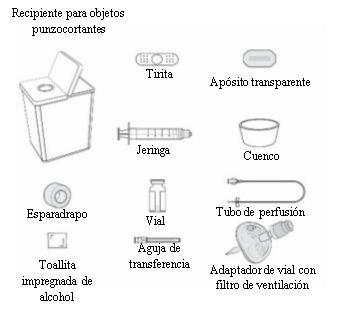 The following materials are not included in the Rystiggo carton:
The following materials are not included in the Rystiggo carton:
- Syringe (5-10 ml, depending on the prescribed dose).
- Transfer needle of 18 gauge or larger or a vial adapter with a ventilation filter.
- Infusion tube with a 26 gauge or larger needle. The infusion tube must be 61 cm or less in length.
- Alcohol swabs.
- Bandage or transparent dressing.
- Cotton ball.
- Container for sharp objects.
- Bowl or absorbent paper to collect any excess liquid when filling the infusion tube.
- Syringe pump (if you are going to use a pump).
! The materials above are for illustrative purposes only. Your specific materials may look different.
Step2:Clean your work surface and wash your hands
- Clean your work surface with a disinfectant and wash your hands thoroughly with soap and water, or use a hand disinfectant. Dry them with a clean towel.
- Prepare the vial and syringe
! Check your dose (you may need more than 1 vial to prepare the prescribed dose).
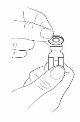
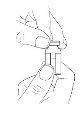 Step3:Remove the protective cap from the vial
Step3:Remove the protective cap from the vial
- Remove the protective cap from the vial by holding the edge and lifting it upwards.
- Clean the rubber stopper with an alcohol swab. Let it air dry.
- Leave the aluminum seal in place.
- Check your dose (if you need more than one vial to prepare the prescribed dose, remove all the caps and clean the stoppers).
! If you are going to use a vial adapter with a ventilation filter instead of a transfer needle, you can go directly to step 7.
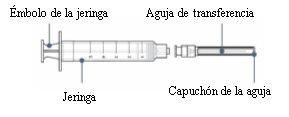
Step4: Connect the transfer needle to the syringe
- Remove the syringe and transfer needle from their respective plastic containers. Do not touch the tip of the syringe or the base of the needle to avoid germs.
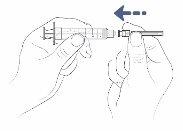 With the needle cap still on, gently push or turn the transfer needle to attach it to the syringe until it is firmly connected.
With the needle cap still on, gently push or turn the transfer needle to attach it to the syringe until it is firmly connected.
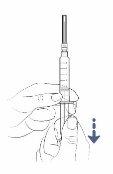 Step5:Draw air into the syringe
Step5:Draw air into the syringe
- Slowly pull the plunger to draw air into the syringe.
- Fill the syringe with approximately the same amount of air as the amount of medication in the vial.
- Keep the needle cap on while doing this.
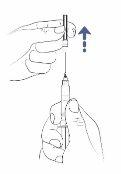
Step6: Remove the transfer needle cap
- Hold the syringe with one hand.
- With the other hand, hold the needle cap and pull it off.
- Place the cap on the table to dispose of it later.
- Do nottouch the needle tip.
- Do notlet the needle tip touch any material after removing the cap.
Step7: Insert the transfer needle or vial adapter with ventilation filter directly into the vial
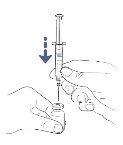 Follow the instructions for the infusion method you are going to use:
Follow the instructions for the infusion method you are going to use:
With transfer needle
- Place the vial on the table and insert the transfer needle directly through the rubber stopper.
 With vial adapter
With vial adapter
- Place the vial on the table and insert the vial adapterdirectly through the rubber stopper.
- Connect the syringe to the vial adapter with a ventilation filter.
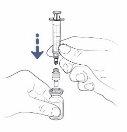
Step8: Turn the vial and syringe
- Now place the vial and syringe upside down.
- Keep the transfer needle or vial adapter with a ventilation filter inside the vial.
! If you are using a vial adapter with a ventilation filter, you can go directly to step 11.
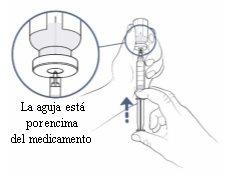 Step9: Push the air from the syringe into the vial
Step9: Push the air from the syringe into the vial
- Check that the transfer needle is pointing upwards and make sure that the needle tip is in the space above the medication.
- Slowly push the syringe plunger upwards to insert all the air from the syringe into the vial. Keep your thumb pushing on the syringe plunger at all times so that air does not enter the syringe.
- Keep the needle tip in the space above the medication at all times.
- Do notpush the air into the medication, as bubbles may form.
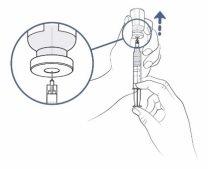
Step10: Prepare to fill the syringe
- Keep your thumb pressing on the syringe plunger. With the other hand, slowly and carefully pull the vial upwards so that the needle tip is completely covered by the liquid medication.
Step11:Fill the syringe with as much medication as possible
! Check your dose (you may need more than 1 vial to prepare the prescribed dose).
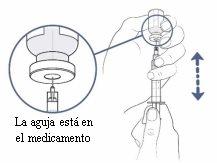 Now slowly pull the syringe plunger downwards and fill the syringe with the medication.
Now slowly pull the syringe plunger downwards and fill the syringe with the medication.
If you are using a transfer needle to fill the syringe, do the following:
- Continue to slowly and carefully pull the vial upwards to keep the needle tip completely covered by the liquid.
- Adjust the needle tip to keep it in the liquid. This will help you extract as much medication as possible from the vial.
- You should now have more medication in the syringe than the prescribed dose. This difference will be adjusted later.
! There will also be a very small amount of medication that you will not be able to extract from the vial. You will need to discard it along with the vial later.
! If you are using a vial adapter with a ventilation filter, disconnect the syringe from the adapter. Leave the vial adapter with a ventilation filter in the vial. You can discard it at the end of the infusion. You can now go directly to step 14.
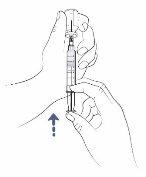
Step12: Remove the air from the syringe
- If there is any space between the liquid in the syringe and the top of the syringe, slowly push the plunger to return the air to the vial.
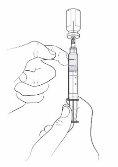
- If you see air bubbles in the syringe, you can remove them by gently tapping the syringe with your index finger. Now slowly push the plunger to return the air to the vial.
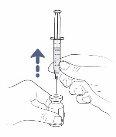
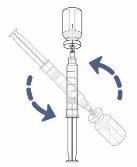
Step13: Remove the transfer needle from the vial and syringe
- Turn the vial and syringe over and place the vial on the work surface.
- Remove the transfer needle and syringe from the vial by pulling the syringe directly upwards.
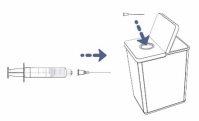 Remove the transfer needle from the syringe by carefully twisting or pulling from the base of the needle.
Remove the transfer needle from the syringe by carefully twisting or pulling from the base of the needle.- Do nottouch the needle. Do notput the cap back on the needle.
- Dispose of the needle in the container for sharp objects.
- If you are using a vial adapter with a ventilation filter instead of a transfer needle, it is not necessary to remove the adapter from the vial before disposing of it.
- If you need to use another vial to prepare the prescribed dose, repeat steps 4-13 with the same syringe and a new transfer needle or a new vial adapter with a vented filter to avoid contamination.
- Prepare for infusion
- Place the syringe on a clean work surface while preparing the infusion tube.
- Remove the infusion tube from the protective bag.
- Remove the cap from the end of the infusion tube by twisting it. Place the cap on the work surface to discard it later.
- Connect the infusion tube to the syringe until they are firmly attached. Do nottouch the tip of the syringe or the base of the infusion tube to avoid germs.
- Do notremove the cap from the needle of the infusion tube.
- Make sure you have a bowl or absorbent paper handy, which you can use to collect any medication from the infusion tube that you do not need.
- Keep the cap on the needle of the infusion tube and hold it over the bowl. Then, hold the syringe in a vertical position and fill the infusion tube with the medication by gently pushing the syringe plunger.
- The amount of liquid left in the syringe should match the prescribed dose.
- If you are using a syringe pump, read the manufacturer's instructions to set up and use the pump, and fill the infusion tube.
- Choose an infusion site on the lower left or right side of your abdomen (below the navel).
- Do notuse a skin area that:
- is sensitive, bruised, red, or hard;
- has scars or stretch marks.
- Prepare the infusion site:
- Clean the infusion site with an alcohol swab and let it air dry.
- Carefully remove the cap from the needle of the infusion tube.
- Fold the butterfly wings to bring them closer and hold them with your thumb and index finger.
- With your other hand, pinch the skin with 2 fingers to create a fold.
- Insert the needle into the center of the skin and push it under the skin.
- The needle should enter easily. If it is difficult, you can withdraw the needle slightly.
- You may be using an infusion tube without a butterfly-type needle at the end. Your nurse or doctor will explain how to insert the needle.
- Use a transparent dressing to keep the needle in place. Some infusion sets have a built-in adhesive.
- You can use tape to secure the infusion tube to the skin.
- Infuse and finish
- Sit comfortably and firmly push the syringe plunger to infuse the medication.
- You should infuse the medication at a speed that feels comfortable for you. Continue pushing until no medication is left in the syringe.
- Before and during the infusion, make sure the infusion tube is not bent or twisted. If this happens, the medication flow may be interrupted. In this case, correct the curvature of the infusion tube and try again.
- If you feel uncomfortable or some of the medication flows back into the infusion tube, you can push more slowly.
- Before using a syringe pump, make sure you understand the following:
- How to set up the syringe pump (set an infusion rate of 20 ml per hour or less).
- How to set the occlusion alarm to the maximum.
- How to start the syringe pump.
- What the different sounds and alarms of the syringe pump mean and what to do in each case.
- How to stop the syringe pump.
- When you are ready for the infusion:
- Place the syringe in its holder and start the pump according to its instructions.
- Sit comfortably while the pump administers the medication to you.
- Before and during the infusion, make sure the infusion tube is not bent or twisted. If this happens, the medication flow may be interrupted. In this case, correct the curvature of the infusion tube and try again.
- Once you have finished, stop the pump according to its instructions.
- Remove the syringe from the pump.
- It is possible that one or two drops of liquid may remain at the infusion site after removing the needle. This is normal.
- Discard the used vial(s) and any remaining medication in the sharps container.
- Cover the infusion site with a clean dressing, such as a band-aid.
- Discard any other used materials in the household trash.
Step14: Recheck your dose
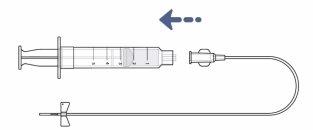 Step 15: Connect the infusion tube to the syringe
Step 15: Connect the infusion tube to the syringe
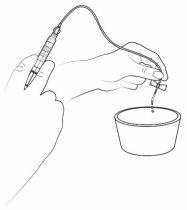
Step 16: Fill the infusion tube with the medication
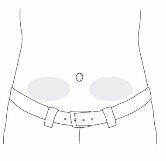
Step 17: Choose and prepare the infusion site
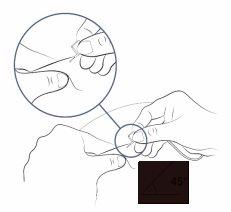 Step 18: Insert the infusion tube needle
Step 18: Insert the infusion tube needle
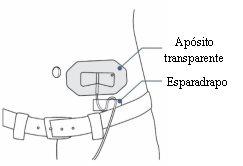 Step 19: Secure the infusion tube needle
Step 19: Secure the infusion tube needle
Step 20:Start the infusion
Follow the instructions for the infusion method you are using:
Manual injection
Syringe pump
! Note: Some medication will remain in the infusion tube. This is normal and can be discarded in the sharps container.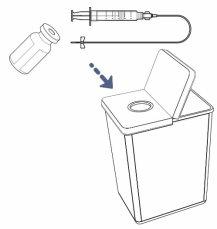
Step 21: Finish the infusion and clean upOnce the infusion is complete, do notattempt to remove the dressing from the needle. Remove both the dressing and the needle from your skin together and discard them with the syringe in the sharps container.
! Always keep the sharps container out of sight and reach of children.
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RYSTIGGO 140 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for RYSTIGGO 140 mg/ml INJECTABLE SOLUTION
Discuss questions about RYSTIGGO 140 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







