REVESTIVE 5 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use REVESTIVE 5 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Revestive 5 mg powder and solvent for solution for injection
teduglutide
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Revestive and what is it used for
- What you need to know before you use Revestive
- How to use Revestive
- Possible side effects
- Storage of Revestive
- Contents of the pack and other information
1. What is Revestive and what is it used for
Revestive contains the active substance teduglutide. It improves the intestinal absorption of nutrients and fluids from your gastrointestinal tract (intestine).
Revestive is used to treat short bowel syndrome in adults, children, and adolescents (from 4 months and older). Short bowel syndrome is a condition where the body is unable to absorb nutrients from food and water as it passes through the intestine. It is usually caused by the surgical removal of a large portion of the small intestine.
2. What you need to know before you use Revestive
Do not use Revestive
- if you are allergic to teduglutide or any of the other ingredients of this medicine (listed in section 6) or to traces of tetracycline residues;
- if you have or think you may have cancer;
- if you have had cancer in the last five years in any part of your gastrointestinal tract, such as the liver, gallbladder, or bile and pancreatic ducts.
Warnings and precautions
Tell your doctor before you start using Revestive:
- if you have severe liver problems. Your doctor will take this into account when prescribing this medicine;
- if you have any heart or blood vessel problems, such as high blood pressure or a weak heart. Signs and symptoms include sudden weight gain, facial swelling, swelling of the ankles and/or difficulty breathing;
- if you have other severe diseases that are not well controlled. Your doctor will take this into account when prescribing this medicine;
- if you have kidney problems. Your doctor may need to give you a lower dose of this medicine.
When you start or while you are receiving treatment with Revestive, your doctor may adjust the amount of fluids or nutrition you receive through a vein.
Medical check-ups before and during treatment with Revestive
Before you start treatment with this medicine, your doctor will need to perform a colonoscopy (a procedure to look inside the colon and rectum) to check for polyps (small abnormal growths) and remove them. It is recommended that your doctor performs these examinations once a year for the first two years after starting treatment and then at least once every five years. If polyps are found before or during treatment with Revestive, your doctor will decide whether you should continue using this medicine. If you are diagnosed with cancer during a colonoscopy, you should not use Revestive. Your doctor will monitor your electrolytes and body fluids, as an imbalance could cause fluid overload or dehydration.
Your doctor will be particularly careful and monitor the function of your small intestine and signs and symptoms that indicate problems in your gallbladder, bile ducts, and pancreas.
Children and adolescents
Medical check-ups before and during treatment with Revestive
Before you start treatment with this medicine, you will have a test to check for blood in your stool. You will also have a colonoscopy (a procedure to look inside the colon and rectum to check for polyps [small abnormal growths] and remove them) if you have blood in your stool of unknown cause. If polyps are found before treatment with Revestive, your doctor will decide whether you should use this medicine. If you are diagnosed with cancer during a colonoscopy, you should not use Revestive. Your doctor will perform more colonoscopies while you are receiving treatment with Revestive. Your doctor will monitor your electrolytes and body fluids, as an imbalance could cause fluid overload or dehydration.
Children under 4 months of age
This medicine must not be used in children under 4 months of age. There is no data available for Revestive in this age group.
Other medicines and Revestive
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
Revestive may affect how other medicines are absorbed in the intestine and reduce their effectiveness. Your doctor may need to change the dose of other medicines.
Pregnancy and breastfeeding
Revestive is not recommended if you are pregnant or breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor, pharmacist, or nurse for advice before using this medicine.
Driving and using machines
This medicine may cause dizziness. If this happens, do not drive or use machines until you feel better.
Important information about some of the ingredients of Revestive
This medicine contains less than 23 mg of sodium (1 mmol) per dose; this is essentially “sodium-free”.
You should be cautious if you are hypersensitive to tetracyclines (see the section “Do not use Revestive”).
3. How to use Revestive
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, ask your doctor, pharmacist, or nurse again.
Dose
The recommended daily dose is 0.05 mg per kg of body weight. The dose will be given in milliliters (ml) of solution.
Your doctor will decide the most suitable dose for you based on your body weight. Your doctor will tell you which dose to inject. If you are unsure, ask your doctor, pharmacist, or nurse again.
Use in children and adolescents
Revestive can be used in children and adolescents (from 4 months or older). Follow the instructions for administration of this medicine exactly as told by your doctor.
How to use Revestive
Revestive is injected under the skin (subcutaneously) once a day. The injection can be given by you or by another person, for example, your doctor, caregiver, or home nurse. If you are injecting the medicine yourself, or your caregiver is injecting it, both you and your caregiver must receive proper training from your doctor or nurse. You will find detailed instructions for injection at the end of this leaflet.
It is strongly recommended that each time you or your child receive a dose of Revestive, you record the name and batch number of the medicine to keep a record of the batches used.
If you use more Revestive than you should
If you accidentally inject more Revestive than your doctor told you to, contact your doctor, pharmacist, or nurse.
If you forget to use Revestive
If you forget to use this medicine (or are unable to use it at the usual time), use it as soon as possible on the same day. Never use more than one injection in the same day. Do not use a double dose to make up for forgotten doses.
If you stop using Revestive
Continue using this medicine for as long as your doctor tells you to. Do not stop using this medicine without talking to your doctor, as sudden stopping may cause changes in your fluid balance.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek medical help immediately if you get any of the following side effects:
Common(may affect up to 1 in 10 people):
- Heart failure. Contact your doctor if you get tired, have difficulty breathing, or swelling of the ankles or legs or facial swelling.
- Pancreatitis (inflammation of the pancreas). Contact your doctor or an emergency service if you get severe stomach pain and fever.
- Intestinal obstruction (blockage of the intestine). Contact your doctor or an emergency service if you get severe stomach pain, vomiting, and constipation.
- Reduced bile flow from the gallbladder and/or inflammation of the gallbladder.
Contact your doctor or an emergency service if you get yellowing of the skin and the white part of the eyes, itching, dark urine, and pale stools or if you get pain in the upper right side or middle of the stomach area.
Uncommon(may affect up to 1 in 100 people):
- Fainting. If your heart rate and breathing are normal and you wake up quickly, tell your doctor. In other cases, seek help as soon as possible.
Other side effects include:
Very common(may affect more than 1 in 10 people):
- Respiratory tract infection (any infection in the nasal passages, throat, airways, or lungs).
- Headache.
- Stomach pain, stomach swelling, feeling of nausea, stoma inflammation, vomiting.
- Redness, pain, or swelling at the injection site.
Common(may affect up to 1 in 10 people):
- Flu or flu-like symptoms.
- Decreased appetite.
- Swelling of hands and/or feet.
- Sleep problems, anxiety.
- Cough, difficulty breathing.
- Polyps (growth of small abnormal masses) in the large intestine.
- Gas (flatulence).
- Narrowing or blockage of the pancreatic duct, which can cause pancreatitis.
- Inflammation of the gallbladder.
Uncommon(may affect up to 1 in 100 people):
- Polyps (growth of small abnormal masses) in the small intestine.
Frequency not known(cannot be estimated from the available data):
- Allergic reaction (hypersensitivity).
- Fluid retention.
- Polyps (growth of small abnormal masses) in the stomach.
Use in children and adolescents
Side effects in children and adolescents are generally similar to those seen in adults.
No data are available for children under 4 months of age.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Revestive
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, vial, and pre-filled syringe after EXP. The expiry date is the last day of the month stated.
Store below 25°C.
Do not freeze.
After reconstitution, from a microbiological point of view, the solution should be used immediately. However, chemical and physical stability has been demonstrated for 3 hours at 25°C.
Do not use this medicine if you notice that the solution is cloudy or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment. All needles and syringes must be disposed of in a sharps container.
6. Container Contents and Additional Information
Revestive Composition
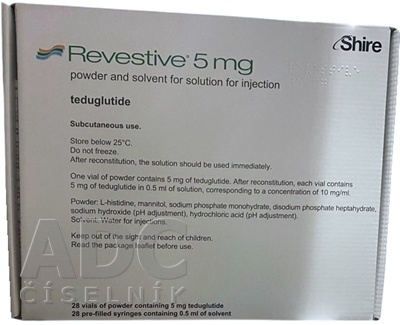
- The active ingredient is teduglutide. One vial of powder contains 5 mg of teduglutide. After reconstitution, each vial contains 5 mg of teduglutide in 0.5 ml of solution, which corresponds to a concentration of 10 mg/ml.
- The other components are L-histidine, mannitol, sodium phosphate monohydrate, and disodium phosphate heptahydrate, sodium hydroxide (pH adjustment), hydrochloric acid (pH adjustment).
- The solvent contains water for injectable preparations.
Product Appearance and Container Contents
Revestive is a powder and solvent for solution for injection (5 mg of teduglutide in a vial, 0.5 ml of solvent in a pre-filled syringe).
The powder is white and the solvent is clear and colorless.
Revestive is available in pack sizes of 1 vial of powder with 1 pre-filled syringe or 28 vials of powder with 28 pre-filled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Takeda Pharmaceuticals International AG Ireland Branch
Block 2 Miesian Plaza
50 – 58 Baggot Street Lower
Dublin 2, D02 HW68
Ireland
Date of Last Revision of this Leaflet: 06/2023
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
Instructions for Preparation and Injection of Revestive
Important Information:
- Read the leaflet before using Revestive.
- Revestive is injected under the skin (subcutaneously).
- Do not inject Revestive into a vein (intravenously) or into a muscle (intramuscularly).
- Keep Revestive out of the sight and reach of children.
- Do not use Revestive after the expiry date stated on the carton, vial, and pre-filled syringe. The expiry date is the last day of the month stated.
- Store below 25°C.
- Do not freeze.
- After reconstitution, from a microbiological point of view, the solution should be used immediately. However, chemical and physical stability has been demonstrated for 3 hours at 25°C.
- Do not use Revestive if the solution is cloudy or contains particles.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
- Dispose of all needles and syringes in a puncture-resistant container.
 Materials included in the package:
Materials included in the package:
- 1 or 28 vials containing 5 mg of teduglutide in powder form.
- 1 or 28 pre-filled syringes with solvent.
Materials needed but not included in the package:
- Reconstitution needles (22G, 1½" [0.7 x 40 mm])
- Injection syringes of 0.5 ml or 1 ml (with interval scales of 0.02 ml or less). For children, a 0.5 ml injection syringe (or smaller) may be used.
- Thin needles for subcutaneous injection (e.g., 26G, 5/8" [0.45 x 16 mm] or smaller needles for children, if applicable).
- Alcohol-impregnated swabs.
- Alcohol-impregnated cotton balls.
- A puncture-resistant container for safe disposal of used syringes and needles.
NOTE:Before starting, wash your hands and ensure you have a clean surface to handle the materials before proceeding.
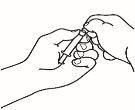
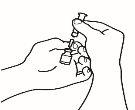
- Assemble the pre-filled syringe
Once all the materials are prepared, you must assemble the pre-filled syringe. The procedure to follow is as follows:
| 1.1 Take the pre-filled syringe with the solvent and remove the top part of the white plastic cap from the pre-filled syringe so that you can attach the reconstitution needle. |
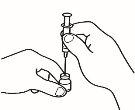
| 1.2 Attach the reconstitution needle (22G 1½" [0.7 x 40 mm]) to the pre-filled syringe already assembled by screwing it in a clockwise direction. |
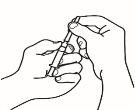
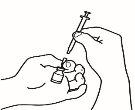
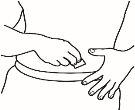
- Dissolve the powder
Now you can dissolve the powder with the solvent.
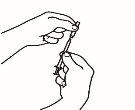
| 2.1 Remove the green seal from the powder vial, clean the top with an alcohol-impregnated swab, and let it dry. Do not touch the top of the vial. |
| 2.2 Remove the needle cap from the reconstitution needle of the pre-filled syringe already assembled with the solvent, without touching the needle tip. |
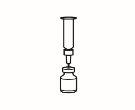
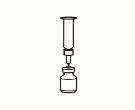
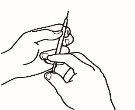
| 2.3 Take the powder vial and insert the reconstitution needle attached to the pre-filled syringe already assembled through the center of the rubber stopper; then carefully press the plunger all the way down to inject all the solvent into the vial. |
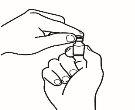
| 2.4 Leave the reconstitution needle and the empty syringe in the vial. Let the contents rest for approximately 30 seconds. |
| 2.5 Gently roll the vial between the palms of your hands for about 15 seconds. Then, carefully invert the vial once, with the reconstitution needle and the empty syringe still in the vial. |
NOTE:Do not shake the vial. Shaking the vial can produce foam, which makes it difficult to extract the solution from the vial.
| 2.6 Let the vial contents rest for about two minutes. |
2.7 Check if the vial contains undissolved powder. If there is still undissolved powder, repeat steps 2.5 and 2.6. Do not shake the vial. If there is still undissolved powder, discard the vial and repeat the procedure from the beginning with a new vial.
NOTE:The final solution should be clear. If the solution is cloudy or contains particles, it should not be injected.
NOTE:Once prepared, the solution should be used immediately. It should be stored below 25°C for a maximum of three hours.
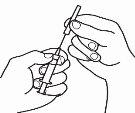
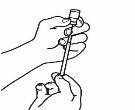
- Prepare the injection syringe
| 3.1 Remove the reconstitution syringe from the reconstitution needle that is still in the vial and discard the reconstitution syringe. |
| 3.2 Take the injection syringe and attach it to the reconstitution needle that is still in the vial. |
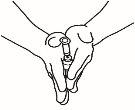
| 3.3 Invert the vial so that it is upside down, slide the tip of the reconstitution needle near the stopper, and gently pull the plunger to fill the syringe with the medicine. |
NOTE:If your doctor has told you that you need two vials, prepare a second pre-filled syringe with solvent and a second vial of powder, as shown in steps 1 and 2. With the same syringe you just used, withdraw the solution from the second vial by repeating step 3.
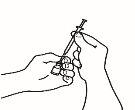
| 3.4 Remove the injection syringe from the reconstitution needle, leaving the needle in the vial. Discard the vial and the reconstitution needle in the puncture-resistant container. |
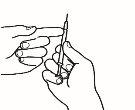
| 3.5 Take the injection needle, but do not remove the plastic cap from the needle. Attach the needle to the injection syringe that contains the medicine. |
| 3.6 Check that there are no air bubbles. If there are, gently tap the syringe with your finger until the bubbles rise to the surface. Then, press the plunger to expel the air bubbles. |
| 3.7 Your doctor has calculated your dose in ml. Expel the excess volume from the syringe with the needle cap in place until you reach the volume of your dose. |
- Inject the solution
|
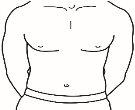
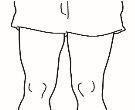
| 4.1 Locate an area of your abdomen where it is easy for you to administer an injection, or your thigh if you have pain or hardening of the abdomen (see the diagram). |
NOTE:Do not always apply injections to the same area; change the area (use the upper, lower, left, or right part of your abdomen) to avoid discomfort. Avoid areas that are inflamed, swollen, scarred, or covered by a mole, birthmark, or any type of lesion.
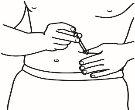
| 4.2 Once you have chosen the injection site, clean the entire area with an alcohol-impregnated cotton ball using circular motions from the inside out. Let the area dry. |
| 4.3 Remove the plastic cap from the needle you have prepared for injection. Gently grasp the clean skin of the injection site with one hand. With the other, hold the syringe like a pencil. Bend your wrist back and quickly insert the needle at a 45-degree angle. |
4.4 Gently pull the plunger. If you see blood in the syringe, remove the needle and replace it in the injection syringe with a new, clean one of the same size. You can still use the medicine inside the syringe. Try to choose another injection site in the same area of clean skin.
4.5 Inject the medicine slowly, pressing the plunger continuously, until you have injected all the medicine and the syringe is empty.
4.6 Quickly remove the needle from the skin and discard the needle and syringe in the puncture-resistant container. You may bleed a little. If necessary, gently press the injection site with an alcohol-impregnated cotton ball or a 2x2 gauze until the bleeding stops.
4.7 Discard all needles and syringes in a puncture-resistant container or a thick-walled container (e.g., a detergent bottle with a cap). You should use a puncture-resistant container or a thick-walled container (including the cap and side walls). If you need a puncture-resistant container, please consult your doctor.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REVESTIVE 5 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: TABLET, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, UnknownActive substance: sodium phenylbutyrateManufacturer: Immedica Pharma AbPrescription requiredDosage form: CAPSULE, 84 mg of eliglustat (as tartrate)Active substance: eliglustatManufacturer: Sanofi B.V.Prescription required
Online doctors for REVESTIVE 5 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about REVESTIVE 5 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions