
PRALUENT 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use PRALUENT 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Praluent 75mg solution for injection in pre-filled syringe
Praluent 150 mg solution for injection in pre-filled syringe
alirocumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Praluent and what is it used for
- What you need to know before you use Praluent
- How to use Praluent
- Possible side effects
- Storage of Praluent
- Contents of the pack and other information
1. What is Praluent and what is it used for
What is Praluent
- The active substance of Praluent is alirocumab.
- Praluent is a monoclonal antibody (a type of specialized protein designed to attach to a target substance in the body). Monoclonal antibodies are proteins that recognize and bind to other unique proteins. Alirocumab binds to PCSK9.
How Praluent works
Praluent helps to reduce your levels of "bad" cholesterol (also called "LDL cholesterol"). Praluent blocks a protein called PCSK9.
- PCSK9 is a protein secreted by liver cells.
- Normally, "bad" cholesterol is removed from the blood by binding to specific "receptors" (docking stations) in the liver.
- PCSK9 reduces the number of these receptors in the liver, which causes "bad" cholesterol levels to be higher than they should be.
- By blocking PCSK9, Praluent increases the number of available receptors to help remove "bad" cholesterol and thus reduces "bad" cholesterol levels.
What Praluent is used for
- In adults with high cholesterol levels in their blood (heterozygous and non-familial hypercholesterolemia or mixed dyslipidemia) and children and adolescents aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH).
- To reduce the risk of cardiovascular events in adults with high cholesterol levels in the blood and with cardiovascular disease.
It is given:
- in combination with a statin (a medicine commonly used to treat high cholesterol) or with other medicines to reduce cholesterol, if the maximum dose of a statin does not sufficiently reduce cholesterol levels, or
- alone or with another cholesterol-reducing medicine when statins are not tolerated or cannot be used.
- Continue with your cholesterol-reducing diet while taking this medicine.
2. What you need to know before you use Praluent
Do not use Praluent
- if you are allergic to alirocumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Praluent.
If you experience a severe allergic reaction, stop treatment with Praluent and go to your doctor immediately. Sometimes, severe allergic reactions have occurred, such as hypersensitivity, including angioedema (difficulty breathing, or swelling of the face, lips, throat, or tongue), nummular eczema (red patches on the skin, sometimes with blisters), and hypersensitivity vasculitis (a specific type of hypersensitivity reaction with symptoms such as diarrhea, with a skin rash, or purple-colored spots on the skin). To learn about allergic reactions that may occur while taking Praluent, see section 4.
Tell your doctor before using this medicine if you have any kidney or liver disease, as Praluent has been studied in few patients with severe kidney disease and in no patients with severe liver disease.
Children and adolescents
Praluent must not be given to children under 8 years of age because there is no experience with the use of this medicine in this age group.
Using Praluent with other medicines
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Praluent is not recommended during pregnancy or breastfeeding.
Driving and using machines
This medicine is not expected to affect your ability to drive or use machines.
3. How to use Praluent
Follow exactly the administration instructions of this medicine given by your doctor, pharmacist, or nurse. If you are unsure, consult your doctor, pharmacist, or nurse again. The outer packaging also contains instructions for use.
How much to inject
Your doctor will tell you what dose is right for you and how often you should inject it (75 mg or 150 mg every 2 weeks or 300 mg every 4 weeks/monthly). Your doctor will check your cholesterol levels and may adjust the dose (increase or decrease) during treatment.
Always check the name and concentration of the medicine on the syringe label.
When to inject
Inject Praluent every 2 weeks (for 75 mg or 150 mg doses) or every 4 weeks/monthly (for 300 mg dose). To administer the 300 mg dose, give two consecutive injections of 150 mg in two different injection sites.
The pre-filled syringes are not intended for use in children and adolescents aged 8 years and older.
Before injecting
Praluent should be allowed to reach room temperature before use.
Read the detailed instructions for use in the package leaflet before injecting Praluent.
Where to inject
Praluent is injected under your skin in the thigh, abdomen, or upper arm.
Read the detailed instructions for use in the package leaflet on where to inject.
How to use the pre-filled syringe
Before using the syringe for the first time, your doctor, pharmacist, or nurse will show you how to inject Praluent.
- Always read the "Instructions for use"included in the box.
- Always use the syringe as described in the "Instructions for use".
If you use more Praluent than you should
If you use more Praluent than you should, consult your doctor, pharmacist, or nurse.
If you forget to use Praluent
If you miss a dose of Praluent, inject the missed dose as soon as possible. Then, inject the next dose according to your regular schedule. This will keep your original treatment schedule. If you are unsure when to inject Praluent, consult your doctor, pharmacist, or nurse.
If you stop treatment with Praluent
Do not stop treatment with Praluent without first talking to your doctor. If you stop treatment with Praluent, your cholesterol levels may increase.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience a severe allergic reaction, stop treatment with Praluent and go to your doctor immediately. Sometimes, severe allergic reactions have occurred, such as hypersensitivity (difficulty breathing), nummular eczema (red patches on the skin, sometimes with blisters), and hypersensitivity vasculitis (a specific type of hypersensitivity reaction with symptoms such as diarrhea, with a skin rash, or purple-colored spots on the skin) (may affect up to 1 in 1,000 people).
Other side effects are:
Common(may affect up to 1 in 10 people)
- redness, itching, swelling, pain/sensitivity at the injection site (local reactions at the injection site)
- signs or symptoms of the upper respiratory tract such as sore throat, nasal discharge, sneezing
- itching (pruritus).
Rare(may affect up to 1 in 1,000 people)
- red, itchy, raised patches on the skin (urticaria).
Frequency not known
Since the marketing of Praluent, the following side effects have been reported, with a frequency not known:
- flu-like illness.
- difficulty breathing, or swelling of the face, lips, throat, or tongue (angioedema).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Praluent
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C to 8°C). Do not freeze.
Keep the syringe in the outer carton to protect it from light.
If necessary, individual pre-filled syringes may be stored below 25°C for a maximum of 30 days. Protected from light. After removal from the refrigerator, Praluent must be used within 30 days or discarded.
Do not use this medicine if it has changed color, is cloudy, or contains visible particles.
After use, put the syringe in a puncture-resistant container. Ask your doctor, pharmacist, or nurse how to dispose of the container. Do not recycle the container.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Praluent Composition
- The active ingredient is alirocumab.
Praluent 75 mg injectable solution in a pre-filled syringe
Each single-use syringe contains 75 milligrams of alirocumab.
Praluent 75 mg injectable solution in a pre-filled syringe
Each single-use syringe contains 150 milligrams of alirocumab.
- The other components are histidine, sucrose, polysorbate 20, and water for injectable preparations.
Product Appearance and Container Contents
Praluent is a clear, colorless to pale yellow injectable solution presented in a pre-filled syringe.
Praluent 75 mg injectable solution in a pre-filled syringe
Each pre-filled syringe with a green plunger contains 1 ml of solution that delivers a single dose of 75 milligrams of alirocumab.
It is available in packs of 1, 2, or 6 pre-filled syringes.
Praluent 150 mg injectable solution in a pre-filled syringeEach pre-filled syringe with a gray plunger contains 1 ml of solution that delivers a single dose of 150 milligrams of alirocumab.
It is available in packs of 1, 2, or 6 pre-filled syringes.
Only some presentations and pack sizes may be marketed.
Marketing Authorization Holder
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly France
Manufacturer
Sanofi Winthrop Industrie
1051 Boulevard Industriel
76580 Le Trait
France
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Sanofi Belgium Tel: +32 (0)2 710 54 00 | Lithuania Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Luxembourg Sanofi Belgium Tel: +32 (0)2 710 54 00 (Belgium) |
Czech Republic Sanofi s.r.o. Tel: +420 233 086 111 | Hungary SANOFI-AVENTIS Zrt., Hungary Tel.: +36 1 505 0050 |
Denmark Sanofi A/S Tlf: +45 45 16 70 00 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Germany Sanofi-Aventis Deutschland GmbH Tel.: 0800 52 52 010 Tel. from abroad: +49 69 305 21 131 | Netherlands Sanofi B.V. Tel: +31 20 245 4000 |
Estonia Swixx Biopharma OÜ Tel: +372 640 10 30 | Norway sanofi-aventis Norge AS Tlf: +47 67 10 71 00 |
Greece Sanofi-Aventis Μονοπρ?σωπη AEBE Τηλ: +30 210 900 16 00 | Austria sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
Spain sanofi-aventis, S.A Tel: +34 93 485 94 00 | Poland Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Call from abroad: +33 1 57 63 23 23 | Portugal Sanofi - Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
Croatia Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Romania Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 | Slovenia Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Iceland Vistor hf. Sími: +354 535 7000 | Slovak Republic Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italy Sanofi S.r.l. Tel: 800 131212 (technical questions) 800 536389 (other questions) | Finland Sanofi Oy Puh/Tel: +358 (0) 201 200 300 |
Cyprus C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sweden Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvia Swixx Biopharma SIA Tel: +371 6 616 47 50 | United Kingdom (Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Praluent Pre-filled Syringe
Instructions for Use
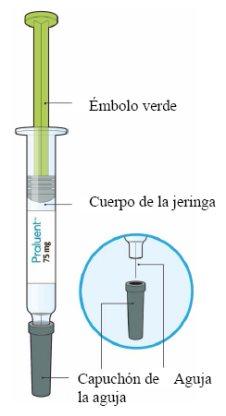
The following image shows the parts of the Praluent pre-filled syringe.


Important Information
- The medicine is injected under the skin and can be administered by you or another person (caregiver).
- This syringe is for single use only and should be discarded after use.
What to Do
- Keep the Praluent pre-filled syringe out of sight and reach of children.
- Read all the instructions carefully before using the Praluent pre-filled syringe.
- Follow these instructions each time you use a Praluent pre-filled syringe.
What Not to Do
- Do not touch the needle.
- Do not use the syringe if it has been dropped or is damaged.
- Do not use the syringe if the gray needle cap is missing or not firmly attached.
- Do not reuse the syringe.
- Do not shake the syringe.
- Do not freeze the syringe.
- Do not expose the syringe to direct sunlight.
Keep this Leaflet. If you have any further questions, ask your doctor, pharmacist, or nurse, or call the sanofi-aventis number listed in the leaflet.
STEP A: Preparing the Injection
Before you start, you will need:
- The Praluent pre-filled syringe
- Alcohol swabs
- A cotton ball or gauze
- A sharps container (see Step B, 6).
Before you start.
- Remove the syringe from the packaging by holding the body of the syringe.
Look at the syringe label.
- Check that it contains the correct medicine and dose (green plunger for 75 mg/ml).
- Check the expiration date on the syringe and do not use it if it is expired.
- Check that the liquid is clear, colorless to pale yellow, and does not contain particles; if not, do not use the syringe.
- Check that the syringe is not open or damaged.
Allow the syringe to reach room temperature for 30 to 40 minutes.
- Let the syringe come to room temperature on its own; do not try to warm it up.
- Do not put the syringe back in the refrigerator.
Prepare the injection site.
- Wash your hands with soap and water and dry them with a towel.
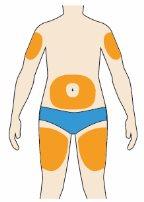
- You can inject into:
- the thigh
- the abdomen (except for the area within 5 cm of the navel)
- the upper outer arm
(See image).
- You can stand or sit while injecting.
- Clean the skin at the injection site with an alcohol swab.
- Do not choose an area of skin that is painful, hardened, red, or inflamed.
- Do not choose an area close to a visible vein.
- Inject each time in a different area.
- Do not inject Praluent together with other injectable medicines in the same area.
STEP B: How to Inject
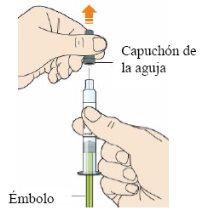
After completing all the steps of “Step A: Preparing the Injection”, remove the needle cap.
- Do not remove the cap until you are ready to inject.
- Hold the middle of the syringe body with the needle facing away from you.
- Do not touch the plunger.
- You may see an air bubble. This is normal. Do not try to remove any air bubbles that may be in the syringe before injection.
- Do not replace the gray cap.
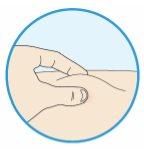
If necessary, pinch the skin.
- Pick up a skin fold at the injection site between your thumb and index finger.
- Do not release the skin fold during the entire injection.
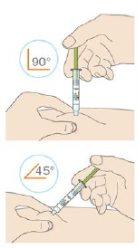
Insert the needle into the skin fold with a quick darting motion.
- If you can pinch 5 cm of skin, use a 90-degree angle.
- If you can only pinch 2 cm of skin, use a 45-degree angle.
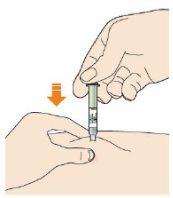
Push the plunger.
- Inject all of the solution by slowly and constantly pushing the plunger.
Before removing the needle, check that the syringe is empty.
- Do not remove the syringe until it is completely empty.
- Remove the needle from the skin at the same angle as you inserted it.
- Do not rub the injection site after the injection.
- If bleeding occurs, press the injection site with a cotton ball or gauze until the bleeding stops.
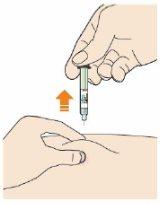
Dispose of the syringe and cap.
- Do not replace the gray needle cap.
- Do not reuse the syringe.
- Dispose of the syringe and cap in a sharps container immediately after use.
- Ask your doctor, pharmacist, or nurse how to dispose of the container.
- Always keep the container out of sight and reach of children.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRALUENT 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 150 mg/mL (1 prefilled syringe)Active substance: alirocumabManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 150 mg/mL (2 prefilled syringes)Active substance: alirocumabManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 150 mg/mL (2 prefilled pens)Active substance: alirocumabManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for PRALUENT 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about PRALUENT 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












