PRADAXA 30 mg GRANULES, COATED


How to use PRADAXA 30 mg GRANULES, COATED
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Pradaxa 20 mg coated granules
Pradaxa 30 mg coated granules
Pradaxa 40 mg coated granules
Pradaxa 50 mg coated granules
Pradaxa 110 mg coated granules
Pradaxa 150 mg coated granules
dabigatran etexilate
Read the entire package leaflet carefully before your child starts taking this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your child's doctor or pharmacist.
- This medicine has been prescribed to your child only, and you should not give it to others, even if they have the same symptoms as your child, as it may harm them.
- If your child experiences side effects, ask your child's doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Pradaxa and what is it used for
- What you need to know before your child starts taking Pradaxa
- How to take Pradaxa
- Possible side effects
- Storage of Pradaxa
- Contents of the pack and further information
1. What is Pradaxa and what is it used for
Pradaxa contains the active substance dabigatran etexilate and belongs to a group of medicines called anticoagulants. It works by blocking a substance in the body involved in the formation of blood clots.
Pradaxa is used in children to treat blood clots and prevent blood clots from forming again.
2. What you need to know before your child starts taking Pradaxa
Do not use Pradaxa
- if your child is allergic to dabigatran etexilate or any of the other ingredients of this medicine (listed in section 6).
- if your child's kidney function is severely reduced.
- if your child is currently bleeding.
- if your child has any disease in an organ of the body that increases the risk of severe bleeding (e.g., stomach ulcer, brain or eye injury, recent brain or eye surgery).
- if your child is prone to bleeding. This tendency may be congenital, of unknown cause, or caused by other medicines.
- if your child is taking medicines to prevent blood clot formation (e.g., warfarin, rivaroxaban, apixaban, or heparin), except when switching anticoagulant treatment or while having a venous or arterial catheter and being administered heparin through this catheter to keep it open.
- if your child's liver function is severely reduced or your child has a life-threatening liver disease.
- if your child is taking ketoconazole or itraconazole orally, medicines used to treat fungal infections.
- if your child is taking cyclosporine orally, a medicine used to prevent organ rejection after a transplant.
- if your child is taking dronedarone, a medicine used to treat abnormal heart rhythm.
- if your child is taking a combination product of glecaprevir and pibrentasvir, an antiviral medicine used to treat hepatitis C.
- if your child has been implanted with an artificial heart valve that requires permanent anticoagulant treatment.
Warnings and Precautions
Ask your child's doctor before starting to give Pradaxa to your child. During treatment with this medicine, your child may also need to consult their doctor if they experience any symptoms or if they need to undergo surgery.
Tell your child's doctorif your child has or has had any disorder or disease, especially any of the following:
- If your child has an increased risk of bleeding, for example:
- if your child has recently had bleeding.
- if your child has had a tissue removal (biopsy) in the last month.
- if your child has had a severe injury (e.g., a bone fracture, a head injury, or any injury that required surgical treatment).
- if your child has inflammation of the esophagus or stomach.
- if your child has problems with gastric juice reflux into the esophagus.
- if your child is taking medicines that may increase the risk of bleeding. See "Other medicines and Pradaxa" below.
- if your child is taking anti-inflammatory medicines such as diclofenac, ibuprofen, or piroxicam.
- if your child has a heart infection (bacterial endocarditis).
- if you know that your child has reduced kidney function or if your child is dehydrated (symptoms include feeling thirsty and passing small amounts of dark-colored urine).
- if your child has a brain or eye infection.
- If your child has had a heart attack or if your child has been diagnosed with diseases that increase the risk of having a heart attack.
- If your child has a liver disease associated with changes in blood tests. The use of this medicine is not recommended in this case.
Be careful with Pradaxa
- If your child needs to undergo surgery:
In this case, Pradaxa should be temporarily discontinued due to an increased risk of bleeding during and shortly after surgery. It is very important that you administer Pradaxa before and after surgery exactly at the times indicated by your child's doctor.
- If surgery requires the placement of a catheter or injection into your child's spinal column (e.g., for epidural or spinal anesthesia or for pain relief):
- It is very important that you administer Pradaxa before and after surgery exactly at the times indicated by your child's doctor.
- Tell your child's doctor immediately if your child experiences numbness or weakness in the legs or bowel or bladder problems after the end of anesthesia, as this situation requires urgent attention.
- If your child falls or is injured during treatment, especially if they hit their head. Seek urgent medical attention. Your child may need to be examined by a doctor, as they may have an increased risk of bleeding.
- If you know that your child has a disease called antiphospholipid syndrome (an immune system disorder that increases the risk of blood clot formation), tell your child's doctor so that they can decide if treatment needs to be modified.
Other medicines and Pradaxa
Tell your child's doctor or pharmacist if your child is taking or has recently taken any other medicines. In particular, you must tell your child's doctor before your child takes Pradaxaif your child is taking any of the following medicines:
- Medicines to reduce blood clotting (e.g., warfarin, phenprocoumon, acenocoumarol, heparin, clopidogrel, prasugrel, ticagrelor, rivaroxaban, aspirin)
- Medicines for the treatment of fungal infections (e.g., ketoconazole, itraconazole), except if only applied to the skin
- Medicines used in the treatment of abnormal heart rhythm (e.g., amiodarone, dronedarone, quinidine, verapamil)
- Medicines for the prevention of organ rejection after a transplant (e.g., tacrolimus, cyclosporine)
- A combination product of glecaprevir and pibrentasvir (an antiviral medicine used to treat hepatitis C)
- Anti-inflammatory and pain-relieving medicines (e.g., aspirin, ibuprofen, diclofenac)
- St. John's Wort, a herbal medicine for depression
- Antidepressant medicines called selective serotonin reuptake inhibitors or selective serotonin and norepinephrine reuptake inhibitors
- Rifampicin or clarithromycin (two antibiotics)
- Antiviral medicines for HIV (e.g., ritonavir)
- Certain medicines for the treatment of epilepsy (e.g., carbamazepine, phenytoin)
Taking Pradaxa with food and drinks
Do not mix Pradaxa coated granules with milk or with soft foods that contain dairy products. Use this medicine only with apple juice or with one of the soft foods indicated in the administration instructions at the end of the package leaflet.
Pregnancy and breastfeeding
This medicine is indicated for use in children under 12 years of age. The information on pregnancy and breastfeeding may not be relevant in the context of treating your child.
The effects of Pradaxa on pregnancy and the fetus are unknown. A pregnant woman should not take this medicine unless her doctor tells her it is safe to do so. A woman of childbearing age should avoid becoming pregnant during treatment with Pradaxa.
Breastfeeding should be interrupted during treatment with Pradaxa.
Driving and using machines
Pradaxa has no known effects on the ability to drive and use machines.
3. How to take Pradaxa
Pradaxa coated granules can be used in children under 12 years of age as soon as they are able to swallow soft foods. Pradaxa capsules are available for the treatment of children 8 years of age or older.
Follow the administration instructions of this medicine exactly as indicated by your child's doctor. If in doubt, consult your child's doctor again.
Pradaxa should be taken twice a day, one dose in the morning and one dose in the evening, approximately at the same time every day. The administration interval should be as close as possible to 12 hours.
The recommended dose depends on weight and age. Your child's doctor will determine the correct dose for your child. It is possible that your child's doctor will adjust the dose during treatment. Your child should continue using all other medicines unless their doctor tells them to stop using any.
Table 1 shows the single and total daily doses of Pradaxa in milligrams (mg) for patients under 12 months of age. The doses depend on the weight in kilograms (kg) and age in months of the patient.
Weight/Age Combinations | Single Dose in mg | Total Daily Dose in mg | |
Weight in kg | Age in MONTHS | ||
2.5 to less than 3 kg | 4 to less than 5 months | 20 | 40 |
3 to less than 4 kg | 3 to less than 6 months | 20 | 40 |
4 to less than 5 kg | 1 to less than 3 months | 20 | 40 |
3 to less than 8 months | 30 | 60 | |
8 to less than 10 months | 40 | 80 | |
5 to less than 7 kg | 0 to less than 1 month | 20 | 40 |
1 to less than 5 months | 30 | 60 | |
5 to less than 8 months | 40 | 80 | |
8 to less than 12 months | 50 | 100 | |
7 to less than 9 kg | 3 to less than 4 months | 40 | 80 |
4 to less than 9 months | 50 | 100 | |
9 to less than 12 months | 60 | 120 | |
9 to less than 11 kg | 5 to less than 6 months | 50 | 100 |
6 to less than 11 months | 60 | 120 | |
11 to less than 12 months | 70 | 140 | |
11 to less than 13 kg | 8 to less than 10 months | 70 | 140 |
10 to less than 12 months | 80 | 160 | |
13 to less than 16 kg | 10 to less than 11 months | 80 | 160 |
11 to less than 12 months | 100 | 200 |
The following are suitable sachet combinations to achieve the recommended single doses in the dosing table. Other combinations are possible.
20 mg: one 20 mg sachet 60 mg: two 30 mg sachets
30 mg: one 30 mg sachet 70 mg: one 30 mg sachet plus one 40 mg sachet
40 mg: one 40 mg sachet 80 mg: two 40 mg sachets
50 mg: one 50 mg sachet 100 mg: two 50 mg sachets
Table 2 shows the single and total daily doses of Pradaxa in milligrams (mg) for patients between 1 year and less than 12 years of age. The doses depend on the weight in kilograms (kg) and age in years of the patient.
Weight/Age Combinations | Single Dose in mg | Total Daily Dose in mg | |
Weight in kg | Age in YEARS | ||
5 to less than 7 kg | 1 to less than 2 years | 50 | 100 |
7 to less than 9 kg | 1 to less than 2 years | 60 | 120 |
2 to less than 4 years | 70 | 140 | |
9 to less than 11 kg | 1 to less than 1.5 years | 70 | 140 |
1.5 to less than 7 years | 80 | 160 | |
11 to less than 13 kg | 1 to less than 1.5 years | 80 | 160 |
1.5 to less than 2.5 years | 100 | 200 | |
2.5 to less than 9 years | 110 | 220 | |
13 to less than 16 kg | 1 to less than 1.5 years | 100 | 200 |
1.5 to less than 2 years | 110 | 220 | |
2 to less than 12 years | 140 | 280 | |
16 to less than 21 kg | 1 to less than 2 years | 110 | 220 |
2 to less than 12 years | 140 | 280 | |
21 to less than 26 kg | 1.5 to less than 2 years | 140 | 280 |
2 to less than 12 years | 180 | 360 | |
26 to less than 31 kg | 2.5 to less than 12 years | 180 | 360 |
31 to less than 41 kg | 2.5 to less than 12 years | 220 | 440 |
41 to less than 51 kg | 4 to less than 12 years | 260 | 520 |
51 to less than 61 kg | 5 to less than 12 years | 300 | 600 |
61 to less than 71 kg | 6 to less than 12 years | 300 | 600 |
71 to less than 81 kg | 7 to less than 12 years | 300 | 600 |
More than 81 kg | 10 to less than 12 years | 300 | 600 |
The following are suitable sachet combinations to achieve the recommended single doses in the dosing table. Other combinations are possible.
50 mg: one 50 mg sachet 140 mg: one 30 mg sachet plus one 110 mg sachet
60 mg: two 30 mg sachets 180 mg: one 30 mg sachet plus one 150 mg sachet
70 mg: one 30 mg sachet plus one 40 mg sachet 220 mg: two 110 mg sachets
80 mg: two 40 mg sachets 260 mg: one 110 mg sachet plus one 150 mg sachet
100 mg: two 50 mg sachets 300 mg: two 150 mg sachets
110 mg: one 110 mg sachet
Form and route of administration
This medicine is administered with apple juice or with one of the soft foods indicated in the administration instructions at the end of the package leaflet. Do not mix this medicine with milk or with soft foods that contain dairy products.
Provided are detailed instructions for the use of this medicine in the "Administration Instructions" section at the end of the package leaflet.
Changing anticoagulant treatment
Do not change your child's anticoagulant treatment without specific instructions from your child's doctor.
If you give more Pradaxa than you should
Taking too much of this medicine increases the risk of bleeding. Contact your child's doctor immediately if you have given too much of this medicine. There are specific treatment options available.
If you forget to give Pradaxa to your child
A forgotten dose can be given up to 6 hours before the next dose.
A forgotten dose should be omitted if the time remaining before the next dose is less than 6 hours.
Do not give a double dose to make up for forgotten doses.
If a dose has been taken only partially, do not try to give a second dose at that time. Give the next dose at the scheduled time, approximately 12 hours later.
If you interrupt treatment with Pradaxa
Give Pradaxa exactly as prescribed. Do not interrupt treatment with this medicine without consulting your child's doctor first, as the risk of blood clot formation may be higher if treatment is interrupted too soon. Contact your child's doctor if your child experiences indigestion after giving Pradaxa.
If you have any other questions about the use of this medicine, ask your child's doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Pradaxa acts on blood coagulation; therefore, most adverse effects are related to signs such as bruising or bleeding. Episodes of major or severe bleeding may occur, which are the most serious adverse effects and, regardless of their location, can cause disability, be potentially life-threatening, or even cause death. In some cases, these bleedings may not be apparent.
If your child experiences any episode of bleeding that does not stop by itself or if your child experiences signs of excessive bleeding (exceptional weakness, fatigue, paleness, dizziness, headache, or unexplained swelling), consult your child's doctor immediately. Your child's doctor may decide to keep your child under close observation or change their medication.
Inform your child's doctor immediately if your child experiences a severe allergic reaction that causes difficulty breathing or dizziness.
Possible adverse effects are detailed below, grouped according to their frequency of occurrence.
Frequent (may affect up to 1 in 10 people):
- Decrease in the number of red blood cells in the blood
- Decrease in the number of platelets in the blood
- Skin rash with dark red, prominent, and itchy bumps, caused by an allergic reaction
- Sudden change in skin color and physical appearance
- Formation of hematomas
- Nosebleeds
- Reflex of gastric juice into the esophagus
- Vomiting
- Feeling like vomiting
- Frequent loose or liquid stools
- Indigestion
- Hair loss
- Increased liver enzymes
Infrequent (may affect up to 1 in 100 people):
- Decrease in the number of white blood cells (which help fight infections)
- Bleeding may occur in the stomach or intestine, brain, rectum, penis/vagina, or urinary tract (including blood in the urine that stains the urine pink or red), or under the skin
- Decrease in the amount of hemoglobin in the blood (the substance present in red blood cells)
- Decrease in the proportion of blood cells
- Itching
- Coughing up blood or sputum with blood spots
- Abdominal pain or stomach pain
- Inflammation of the esophagus and stomach
- Allergic reaction
- Difficulty swallowing
- Yellowish discoloration of the skin or the whites of the eyes, caused by liver or blood problems
Frequency not known (frequency cannot be estimated from available data):
- Lack of white blood cells (which help fight infections)
- Severe allergic reaction that causes difficulty breathing or dizziness
- Severe allergic reaction that causes swelling of the face or throat
- Difficulty breathing or wheezing
- Bleeding
- Bleeding may occur in a joint or wound, in a surgical incision, at the site of injection, or at the site of a catheter in a vein
- Bleeding may be from hemorrhoids
- Ulcer in the stomach or intestine (including ulcer in the esophagus)
- Abnormalities in liver function tests
Reporting Adverse Effects
If your child experiences any type of adverse effect, consult your doctor or pharmacist, even if it is an adverse effect that does not appear in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Pradaxa
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the box after "EXP". The expiration date is the last day of the month indicated.
Before the first use, do not open the aluminum bag that contains the sachets with Pradaxa coated granules to protect it from moisture.
Once the aluminum bag that contains the sachets with the coated granules and the desiccant is opened, the medicine must be used within the next 6 months. The opened sachet cannot be stored and must be used immediately after opening.
Medicines should not be thrown down the drain. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Pradaxa
- The active ingredient is dabigatran. Each sachet of Pradaxa 20 mg coated granules contains coated granules with 20 mg of dabigatran etexilate (in the form of mesylate).
- The active ingredient is dabigatran. Each sachet of Pradaxa 30 mg coated granules contains coated granules with 30 mg of dabigatran etexilate (in the form of mesylate).
- The active ingredient is dabigatran. Each sachet of Pradaxa 40 mg coated granules contains coated granules with 40 mg of dabigatran etexilate (in the form of mesylate).
- The active ingredient is dabigatran. Each sachet of Pradaxa 50 mg coated granules contains coated granules with 50 mg of dabigatran etexilate (in the form of mesylate).
- The active ingredient is dabigatran. Each sachet of Pradaxa 110 mg coated granules contains coated granules with 110 mg of dabigatran etexilate (in the form of mesylate).
- The active ingredient is dabigatran. Each sachet of Pradaxa 150 mg coated granules contains coated granules with 150 mg of dabigatran etexilate (in the form of mesylate).
- The other components are tartaric acid, arabic gum, hypromellose, dimethicone 350, talc, and hydroxypropylcellulose.
Appearance of the Product and Package Contents
The sachets of Pradaxa coated granules contain a yellowish granulate.
Each package of this medicine contains an aluminum bag that, in turn, contains 60 silver aluminum sachets with Pradaxa coated granules and a desiccant (with the inscription "DO NOT EAT" and a pictogram and the inscription "SILICA GEL").
Marketing Authorization Holder
Boehringer Ingelheim International GmbH
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
Manufacturer
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium Boehringer Ingelheim SCommTel/Tel: +32 2 773 33 11 | Lithuania Boehringer Ingelheim RCV GmbH & Co KG Lithuanian branch Tel: +370 5 2595942 |
Bulgaria Boehringer Ingelheim Bulgaria EOOD Tel: +359 2 958 79 98 | Luxembourg Boehringer Ingelheim SCommTel/Tel: +32 2 773 33 11 |
Czech Republic Boehringer Ingelheim spol. s r.o. Tel: +420 234 655 111 | Hungary Boehringer Ingelheim RCV GmbH & Co KG Hungarian Branch Office Tel: +36 1 299 8900 |
Denmark Boehringer Ingelheim Danmark A/S Tlf: +45 39 15 88 88 | Malta Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 |
Germany Boehringer Ingelheim Pharma GmbH & Co. KG Tel: +49 (0) 800 77 90 900 | Netherlands Boehringer Ingelheim B.V. Tel: +31 (0) 800 22 55 889 |
Estonia Boehringer Ingelheim RCV GmbH & Co KG Estonian branch Tel: +372 612 8000 | Norway Boehringer Ingelheim Norway KS Tlf: +47 66 76 13 00 |
Greece Boehringer Ingelheim Hellas Single Member S.A. Tel: +30 2 10 89 06 300 | Austria Boehringer Ingelheim RCV GmbH & Co KG Tel: +43 1 80 105-7870 |
Spain Boehringer Ingelheim España S.A. Tel: +34 93 404 51 00 | Poland Boehringer Ingelheim Sp.zo.o. Tel: +48 22 699 0 699 |
France Boehringer Ingelheim France S.A.S. Tél: +33 3 26 50 45 33 | Portugal Boehringer Ingelheim Portugal, Lda. Tel: +351 21 313 53 00 |
Croatia Boehringer Ingelheim Zagreb d.o.o. Tel: +385 1 2444 600 | Romania Boehringer Ingelheim RCV GmbH & Co KG Vienna - Bucharest Branch Tel: +40 21 302 2800 |
Ireland Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 | Slovenia Boehringer Ingelheim RCV GmbH & Co KG Ljubljana Branch Office Tel: +386 1 586 40 00 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovak Republic Boehringer Ingelheim RCV GmbH & Co KG organizational unit Tel: +421 2 5810 1211 |
Italy Boehringer Ingelheim Italia S.p.A. Tel: +39 02 5355 1 | Finland Boehringer Ingelheim Finland Ky Tel: +358 10 3102 800 |
Cyprus Boehringer Ingelheim Hellas Single Member S.A. Tel: +30 2 10 89 06 300 | Sweden Boehringer Ingelheim AB Tel: +46 8 721 21 00 |
Latvia Boehringer Ingelheim RCV GmbH & Co KG Latvian branch Tel: +371 67 240 011 | United Kingdom (Northern Ireland) Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 |
Date of Last Approval of this Leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
Administration Instructions
Do not administer Pradaxa coated granules
- through syringes or feeding tubes
- with other foods or drinks that are not apple juice and soft foods as indicated below
Administer Pradaxa coated granules with soft foods or with apple juice. The instructions are provided below in sections A) for soft foods and B) for apple juice.
The prepared medicine must be administered before meals to ensure that the patient takes the complete dose.
Administer the prepared medicine to the patient immediately or within 30 minutes of mixing. Do not administer this medicine if it has been in contact with food or apple juice for more than 30 minutes.
In case of incomplete ingestion of the prepared medicine, do not administer a second dose; wait for the next administration time.
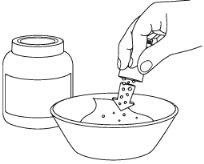
- Administration of Pradaxa Coated Granules with Soft Foods
The food must be at room temperature before mixing it with the coated granules. The medicine can be administered with one of the following soft foods:
- carrot puree
- apple compote (for administration with apple juice, see B)
- banana puree
Do not use soft foods that contain dairy products.
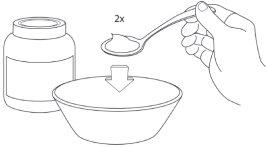
Step 1: Prepare a cup or bowl
|
|
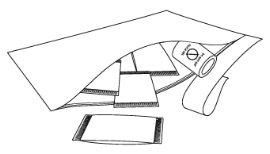
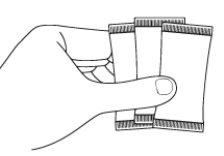
Step 2: Take the sachet(s)
|
|
|
|
|
|
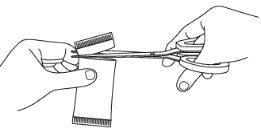
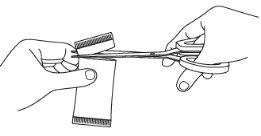
Step 3: Open the sachet(s)
|
|
Step 4: Pour the sachet(s)
|
|
Step 5: Mix the soft food to combine the coated granules
|
|
Step 6: Administer the soft food
|
|
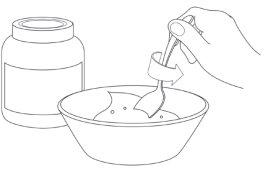
- Administration of Pradaxa Coated Granules with Apple Juice
Step 1: Have a cup of apple juice ready before the next step
Step 2: Take the sachet(s)
|
|
|
|
|
|
Step 3: Open the sachet(s)
|
|
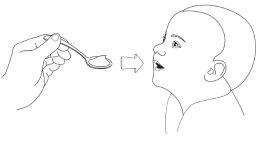
Step 4: Administer Pradaxa Coated Granules with Apple Juice
- Administer all the coated granules directly from the sachet or using a feeding spoon in the child's mouth and offer the child the amount of apple juice needed to swallow the coated granules.
- Check the child's mouth to ensure that all the coated granules have been swallowed.
- Optional: If Pradaxa coated granules are mixed in the cup of apple juice, start with a small amount of apple juice (that the child is likely to drink completely) and make sure that the child takes all the coated granules. If the coated granules stick to the cup, add another small amount of apple juice and give it to the child again. Repeat the process until no coated granules are stuck to the cup.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRADAXA 30 mg GRANULES, COATEDDosage form: CAPSULE, 110 mgActive substance: dabigatran etexilateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: CAPSULE, 150 mgActive substance: dabigatran etexilateManufacturer: Adamed Laboratorios S.L.U.Prescription requiredDosage form: CAPSULE, 75 mgActive substance: dabigatran etexilateManufacturer: Adamed Laboratorios S.L.U.Prescription required
Online doctors for PRADAXA 30 mg GRANULES, COATED
Discuss questions about PRADAXA 30 mg GRANULES, COATED, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions