How to use Zivafert
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: patient information
Zivafert, 5000 IU
powder and solvent for solution for injection
Chorionic gonadotropin
This medicinal product is subject to additional monitoring. This will allow for quick
identification of new safety information. The medicine user can also help by reporting any
adverse reactions that occur after taking the medicine. To learn how to report adverse reactions – see section 4.
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any adverse reactions, including any adverse reactions not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Adverse reactions not listed in this leaflet should be reported to the doctor or pharmacist.
See section 4.
- This leaflet refers to the medicine Zivafert 5000 IU powder and solvent for solution for injection as Zivafert.
Table of contents of the leaflet
- 1. What is Zivafert and what is it used for
- 2. Important information before taking Zivafert
- 3. How to take Zivafert
- 4. Possible side effects
- 5. How to store Zivafert
- 6. Contents of the pack and other information
1. What is Zivafert and what is it used for
What is Zivafert:
Zivafert contains highly purified human chorionic gonadotropin, a hormone obtained from human urine, belonging to the family of "gonadotropins" which are natural hormones responsible for reproduction and fertility.
When to use Zivafert:
Zivafert is used to:
- stimulate the development and maturation of several follicles (each containing an egg cell) in women undergoing assisted reproduction techniques (a procedure that can help get pregnant), such as "in vitro fertilization";
- stimulate the release of an egg from the ovary (induction of ovulation) in women who are unable to produce egg cells ("anovulation") or women who produce too few egg cells ("oligo-ovulation").
This medicine should be used under the supervision of a doctor, unless the doctor advises otherwise.
2. Important information before taking Zivafert
When not to use Zivafert:
- if the patient is allergic to human chorionic gonadotropin or any of the other ingredients of this medicine (listed in section 6);
- if the patient has untreated diseases of the thyroid, pituitary or adrenal glands;
- if the patient has cancer of the ovary, uterus or breast;
- if the patient has a condition that prevents normal pregnancy, such as ovarian failure, absence of the uterus, premature menopause, blocked fallopian tubes, uterine fibroids or other abnormalities of the reproductive organs;
- if the patient has recently experienced unexplained vaginal bleeding.
Tell your doctor or pharmacist if you have any of the above conditions, as this medicine may not be suitable for you.
Warnings and precautions
Before starting treatment, your doctor should check that your reproductive organs are normal.
Before starting Zivafert, discuss with your doctor if you have or have had any of the following conditions:
- abnormalities of the reproductive organs;
- chronic diseases (such as diabetes, cardiovascular disorders, etc.);
- vascular complications (i.e. increased risk of blood clots, history of blood clots in you or your family, overweight).
Medical examinations
Up to 10 days after administration of Zivafert, a pregnancy test may give a false positive result.
During treatment with Zivafert, the following may occur:
Ovarian Hyperstimulation Syndrome (OHSS)
Treatment with gonadotropin hormones, such as Zivafert, may cause OHSS. This is a serious condition in which the ovaries are over-stimulated, and the developing follicles become too large. In rare cases, OHSS can be life-threatening. Therefore, it is very important that you remain under close supervision of your doctor. Your doctor will perform an ultrasound examination (USG) of the ovaries to check the effects of treatment. They may also recommend monitoring hormone levels in your blood (see also section 4).
In OHSS, fluid accumulates very quickly in the abdominal cavity and chest. Blood clots may also form. You should immediately consult your doctor if you experience:
- significant abdominal swelling and abdominal pain;
- nausea;
- vomiting;
- sudden weight gain due to fluid accumulation;
- diarrhea;
- reduced urine output;
- breathing difficulties.
Ovarian torsion
Ovarian torsion is a condition in which the ovary becomes twisted, which can cut off blood flow to the ovary.
Before starting Zivafert, tell your doctor if:
- you have ever had OHSS;
- you are pregnant or think you may be pregnant;
- you have had abdominal surgery;
- you have had ovarian torsion;
- you have had ovarian cysts.
Blood clots (thrombosis)
Pregnancy increases the risk of blood clots.
If you have risk factors for blood clots (such as overweight or blood clots in your family), the risk of blood clots in a blood vessel may increase during in vitro fertilization treatment.
Blood clots can be associated with serious conditions, such as:
- pulmonary embolism;
- stroke;
- heart attack;
- reduced blood flow to vital organs, which can lead to organ damage;
- reduced blood flow (deep vein thrombosis) to the arms or legs, which can lead to amputation of the arm or leg.
Multiple pregnancies, birth defects, miscarriage, or pregnancy complications
Pregnancies after Zivafert treatment are more likely to be twin or multiple pregnancies.
Multiple pregnancies are associated with an increased risk for the mother and child during the perinatal period.
In women undergoing infertility treatment, there is a slightly increased risk of miscarriage or ectopic pregnancy (a pregnancy that develops outside the uterus). Therefore, your doctor should perform an early ultrasound examination to rule out the possibility of an ectopic pregnancy. Multiple births are more likely if you are taking other ovulation-inducing drugs (e.g. hMG).
It is not known whether in vitro fertilization treatment causes congenital malformations of the fetus or the occurrence of certain genital tumors.
Children and adolescents
Zivafert is not indicated for use in children and adolescents.
Zivafert and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take, including those available without a prescription. This is especially important if you are taking medicines that:
- stimulate ovulation (e.g. hMG);
- contain corticosteroids, especially in high doses.
Pregnancy and breastfeeding
Do not use Zivafert if you are pregnant or breastfeeding. If you think you may be pregnant, consult your doctor before taking this medicine.
Driving and using machines
Zivafert has no influence on the ability to drive and use machines.
Zivafert contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per injection, which means it is essentially "sodium-free".
3. How to take Zivafert
Zivafert is a powder that must be dissolved in a liquid (solvent) before use; it is administered by injection under the skin (subcutaneously) or into a muscle (intramuscularly). The solution is prepared by combining the solvent with the powder and should be used immediately after preparation.
This medicine should always be used exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist.
Method of administration
How to administer Zivafert:
Zivafert is administered by injection under the skin (subcutaneously) or into a muscle (intramuscularly).
Each vial should only be used once, and the injection should be administered immediately after preparing the solution.
The recommended dose of Zivafert is 5,000 IU or 10,000 IU. The medicine should be administered within 24-48 hours of optimal follicular growth stimulation.
After providing the patient with appropriate counseling and training, the doctor may recommend that the patient administer Zivafert themselves.
Before the first self-administration of Zivafert, the doctor:
- will explain how to prepare the correct dose of the medicine;
- will show how to prepare the solution for injection;
- will show the possible injection sites;
- will allow the patient to practice self-administering the subcutaneous injection.
Before self-administering Zivafert, read the following instructions carefully.
How to prepare the Zivafert solution from 1 vial of powder:
The solution should be prepared just before injection. Each vial is for single use only.
Zivafert can only be prepared with the solvent provided in the packaging, according to the following instructions:
Step 1
Wash your hands before preparing the solution. Use a clean surface. It is essential to keep your hands and the objects you use as clean as possible.
Step 2
Lay out the following items on a clean surface:
- 2 alcohol swabs (not included in the kit);
- 1 vial containing Zivafert powder;
- 1 solvent ampoule-syringe;
- 1 long needle for reconstitution and intramuscular injection;
- 1 short needle for subcutaneous injection.
Step 3
- Remove only the cap from the ampoule-syringe.
- Put the reconstitution needle (long needle) with the protective cap on the syringe and check that the needle is properly seated to avoid leakage of the solution. If the solution leaks, try to adjust the needle connection by gently turning it.
- Carefully place the syringe on a clean surface.
- Avoid touching the needle.
Step 4
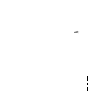
- Remove the colored, plastic cap from the Zivafert vial by gently pushing it upwards.
- Wipe the rubber stopper with an alcohol swab and let it dry.
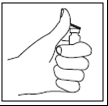
Step 5
- Pick up the syringe, remove the protective cap from the needle, and insert the needle through the rubber stopper in the top of the Zivafert vial.
- Press the plunger firmly and slowly inject the solvent into the vial with the powder to dissolve the entire contents.
- DO NOT SHAKE, gently swirl the vial in your hands until the powder is completely dissolved, taking care not to create foam.
Step 6
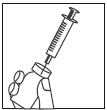
After the powder has dissolved (which usually happens immediately), slowly draw up the solution into the syringe as follows:
- Leaving the needle in place, turn the vial upside down.
- Make sure the tip of the needle is below the level of the liquid.
- Gently pull the plunger to draw the entire Zivafert solution into the syringe.
- Check that the reconstituted solution is clear and colorless.
Preparing larger doses using more than 1 vial of powder:
If your doctor has prescribed a higher dose of Zivafert – 10,000 IU, this can be achieved by using two vials of powder and one ampoule-syringe with solvent.
When preparing 2 vials of Zivafert, at the end of the above step 3, draw up the reconstituted contents of the first vial into the syringe and slowly inject it into the second vial.
Repeat steps 4 to 6 for the second vial.
The solution must be clear and colorless.
Intramuscular injection:
In the case of intramuscular injection, the doctor or nurse will prepare and then administer Zivafert into the thigh or buttock.
Subcutaneous injection:
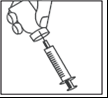
- When the syringe contains the correct dose, put the protective cap on the long needle. Remove the long needle from the syringe and replace it with the short needle for subcutaneous injection with the protective cap in place. Check that the needle is properly seated and press the short needle firmly onto the syringe cylinder, then gently turn it to ensure it is fully secured, to avoid leakage of the solution.
- Remove the protective cap from the needle. Hold the syringe with the needle facing upwards and gently tap the side of the syringe to bring any air bubbles to the top.
- Press the plunger until a drop of liquid appears at the tip of the needle.
- DO NOT USE the solution if it contains any particles or is cloudy.
- Your doctor or nurse will advise you on where to inject the medicine. Typical injection sites are the thighs or abdomen (below the navel).
- Wipe the injection site with an alcohol swab.
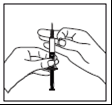
- Firmly pinch the skin. With your other hand, insert the needle at a 45° or 90° angle.
Injection site:
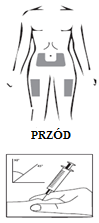
Injecting the solution:
- Inject the solution under the skin as directed by your doctor.
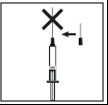
- Do not inject the solution directly into a vein.
- Slowly and evenly press the plunger to ensure the solution is administered correctly and the skin tissue is not damaged.
Take your time to inject the prescribed volume of the solution.
Removing the needle:
- Quickly withdraw the syringe and press the injection site.
- Gently massage the injection site – while maintaining pressure – to help distribute the Zivafert solution and alleviate discomfort.
Disposal of all used items:
After the injection, dispose of all needles and empty syringes in an appropriate container. Dispose of any unused solution or waste according to local regulations.
Using more than the recommended dose of Zivafert
The effects of overdosing with Zivafert are not known; however, it can be expected that ovarian hyperstimulation syndrome (OHSS) may occur (see "Possible side effects"). If you have taken more than the recommended dose of Zivafert, contact your doctor or pharmacist.
Missing a dose of Zivafert
If you miss a dose of Zivafert, contact your doctor immediately.
Stopping Zivafert treatment
If you do not intend to use this medicine, consult your doctor.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Zivafert can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop taking Zivafert and seek medical help immediately:
- mild or moderate ovarian hyperstimulation syndrome (OHSS), which may cause ovarian enlargement, ovarian cysts, abdominal pain, nausea, and vomiting (see also section 2, "Ovarian Hyperstimulation Syndrome"). This is a common side effect (may affect up to 1 in 10 people).
- severe ovarian hyperstimulation syndrome (OHSS) characterized by abdominal pain (pelvic pain), nausea, vomiting, weight gain, fluid accumulation in the abdominal cavity (ascites) or chest (pleural effusion). This is an uncommon side effect (may affect up to 1 in 100 people).
- rupture of an ovarian cyst (as a rare complication of severe OHSS, may affect up to 1 in 1,000 people).
- formation of blood clots in blood vessels (thromboembolic events) as a complication of OHSS. This is a rare side effect (may affect up to 1 in 1,000 people).
- severe generalized allergic reactions, which may include: facial swelling, eye swelling, lip swelling, throat or tongue swelling, difficulty breathing, wheezing, skin rash. This is a rare side effect (may affect up to 1 in 1,000 people).
Other side effects:
Common side effects(may affect up to 1 in 10 people):
- reaction at the injection site, which may include redness, bruising, swelling, itching, or pain at the injection site;
- edema;
- mood changes;
- headache;
- breast tenderness.
Uncommon side effects(may affect up to 1 in 100 people):
- restlessness (agitation);
- fatigue.
Rare side effects(may affect up to 1 in 1,000 people):
- swelling in the deep layers of the skin, often with hives;
- widespread skin rash.
If you experience any of the rare side effects listed above, seek medical help immediately, as your doctor will assess whether you should stop taking Zivafert or seek immediate hospital attention.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw,
Phone: +48 22 49 21 301,
Fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Zivafert
Keep the medicine out of the sight and reach of children.
Do not store above 25°C. Store the vial and ampoule-syringe with solvent in the outer packaging to protect from light.
Use the solution immediately after preparation.
Do not use this medicine after the expiry date stated on the carton, vial, ampoule-syringe with solvent after: EXP. If the expiry date is stated as a month and year, the expiry date refers to the last day of the stated month.
Do not use this medicine if you notice that the solution is not clear (cloudy or contains visible particles). After reconstitution, the solution must be clear and colorless.
Medicines should not be disposed of via wastewater. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Zivafert contains
- The active substance is human chorionic gonadotropin.
- The other ingredients are:
- vial with powder: lactose monohydrate;
- ampoule-syringe with solvent (0.9% sodium chloride solution): water for injections, sodium chloride.
Each vial contains: human chorionic gonadotropin 5000 IU, obtained from human urine.
What Zivafert looks like and contents of the pack
Zivafert is available as:
Powder in a vial: lyophilized powder, white or almost white.
Solvent in an ampoule-syringe (0.9% sodium chloride solution): clear and colorless solution.
A single pack contains:
A plastic tray containing a vial of powder made of clear glass type I, closed with a rubber stopper and an aluminum flip-off cap.
1 ml of solvent in an ampoule-syringe made of clear glass type I with a rubber stopper (also serving as a plunger) and an isoprene and bromobutyl cap, and 1 injection needle for reconstitution and intramuscular injection, and 1 injection needle for subcutaneous injection.
A multipack (10+10) contains 10 plastic trays as described above.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
IBSA Farmaceutici Italia s.r.l.
Via Martiri di Cefalonia 2
26900 Lodi
Italy
[email protected]
Importer:
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia 2
26900 Lodi
Italy
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
AT:
Zivafert
DK:
Gonasi Set
CZ:
Zivafert
EL:
Zivafert
ES:
Gonasi Kit
FI:
Gonasi Set
FR:
GONADOTROPHINE CHORIONIQUE IBSA
HU:
Zivafert Kit
NL:
Gonasi
NO:
Gonasi Set
PL:
Zivafert
SE:
Gonasi Set
SK:
Gonasi Kit
Date of last revision of the leaflet:January 2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterIBSA Farmaceutici Italia S.r.l.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZivafertDosage form: Powder, 1500 IUActive substance: chorionic gonadotrophinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 5000 IUActive substance: chorionic gonadotrophinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 75 IUActive substance: urofollitropinManufacturer: Imed Poland Sp. z o.o. Laboratoires Genevrier S.A.Prescription required
Alternatives to Zivafert in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Zivafert in Ukraine
Alternative to Zivafert in Spain
Online doctors for Zivafert
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Zivafert – subject to medical assessment and local rules.