
GONASI KIT 5,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use GONASI KIT 5,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Gonasi Kit 5,000 IUpowder and solvent for solution for injection
human chorionic gonadotropin
?This medicinal product is subject to additional monitoring, which will allow for quicker identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- In this leaflet, Gonasi Kit 5,000 IU powder and solvent for solution for injection is referred to as Gonasi Kit.
Contents of the pack
- What Gonasi Kit is and what it is used for
- What you need to know before you use Gonasi Kit
- How to use Gonasi Kit
- Possible side effects
- Storage of Gonasi Kit
- Contents of the pack and other information
1. What Gonasi Kit is and what it is used for
WhatGonasi Kitis
This medicinal product contains highly purified human chorionic gonadotropin, a hormone obtained from human urine and belonging to the family of "gonadotropins", which are natural hormones related to reproduction and fertility.
What Gonasi Kit is used for:
This medicinal product is used:
- To help develop and mature several follicles (each containing an egg) in women undergoing assisted reproduction techniques (a procedure that can help you get pregnant), such as "in vitro fertilization" (IVF)
- To help release an egg from the ovary (induction of ovulation) in women who cannot produce eggs ("anovulation") or produce very few ("oligovulation").
This medicinal product should be used under the supervision of your doctor, unless otherwise indicated by your doctor.
2. What you need to know before you use Gonasi Kit
Do not useGonasi Kit:
- if you are allergic to human chorionic gonadotropin or to any of the other ingredients of this medicinal product (listed in section 6)
- if you have untreated diseases that affect the thyroid, pituitary or adrenal glands
- if you have ovarian, uterine or breast cancer
- if you have a condition that makes a normal pregnancy impossible, for example, ovarian failure, absence of uterus, premature menopause, obstruction of the fallopian tubes, uterine fibroids or other anomalies of the reproductive organs
- if you have recently had vaginal bleeding of unknown cause.
Consult your doctor or pharmacist if any of the above conditions apply to you, as this medicinal product may not be suitable for you.
Warnings and precautions
Your doctor should check, before treatment, that your reproductive organs are normal.
Consult your doctor before using this medicinal product if you have or have had any of the following conditions:
- anomalies of the reproductive organs
- long-term disease (such as diabetes, cardiovascular disorders, etc.).
- vascular complications (i.e., increased risk of blood clots, family history or previous blood clots, overweight).
Medical examinations
During a period of up to ten days after administration of this medicinal product, a pregnancy test may give a false positive result.
During treatment with this medicinal product, the following may occur:
Ovarian Hyperstimulation Syndrome (OHSS)
Treatment with gonadotropin hormones like this medicinal product can cause Ovarian Hyperstimulation Syndrome (OHSS). This is a serious condition in which the ovaries are overstimulated and the growing follicles become larger than normal. In rare cases, severe OHSS can be life-threatening. Therefore, close supervision by your doctor is very important. To check the effects of treatment, your doctor will perform ultrasound scans of your ovaries. Your doctor may also check hormone levels in your blood. (See also section 4, "Possible side effects").
OHSS causes fluid to accumulate suddenly in the abdominal (stomach) and chest areas, which can cause blood clots to form. Inform your doctor immediately if you have:
- severe swelling and pain in the stomach area (abdomen)
- nausea
- vomiting
- sudden weight gain due to fluid accumulation
- diarrhea
- decreased urine output
- difficulty breathing.
Ovarian torsion
Ovarian torsion is the twisting of an ovary. The twisting of the ovary could cut off blood flow to the ovary.
Before starting treatment with this medicinal product, it is important that you inform your doctor if:
- you have ever had OHSS
- you are pregnant or think you may be pregnant
- you have had abdominal surgery
- you have had ovarian torsion
- you have cysts or have had cysts on your ovary or ovaries.
Blood clots (thrombosis)
Pregnancy increases the likelihood of blood clots forming.
If you have risk factors for blood clots (e.g., overweight, or if you have a family history of blood clots), the likelihood of a blood clot forming in a blood vessel may increase during IVF treatment.
Blood clots can cause serious conditions, such as:
- blockage in your lungs (pulmonary embolism)
- stroke
- heart attack
- reduced blood flow to vital organs that can cause organ damage
- reduced blood flow (deep vein thrombosis) in your arm or leg that can cause loss of your arm or leg.
Multiple births, birth defects, miscarriages, or pregnancy complications
If treatment with this medicinal product results in pregnancy, there is a higher likelihood of having twins or multiple births. Multiple pregnancies pose an increased risk to the health of both the mother and the babies, before and after birth. In women undergoing fertility treatment, there is a slightly higher risk of miscarriage or ectopic pregnancy (a pregnancy outside the uterus). Therefore, your doctor should perform an ultrasound scan at the beginning to rule out the possibility of an ectopic pregnancy. Multiple births are more likely if you are taking other medicines that stimulate ovulation (e.g., hMG).
It is not known whether IVF treatment causes congenital malformations or certain cancers of the sex organs.
Children and Adolescents
This medicinal product should not be used in children or adolescents.
Other medicines and Gonasi Kit
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription. This is especially important if you are taking medicines that:
- stimulate ovulation (e.g., hMG);
- contain corticosteroids, especially in high doses.
Pregnancy and breast-feeding
Do not use this medicinal product if you are pregnant or breast-feeding. If you think you may be pregnant, consult your doctor before using this medicinal product.
Driving and using machines
This medicinal product does not affect your ability to drive or use machines.
Gonasi Kit contains sodium
This medicinal product contains less than 1 mmol of sodium (23 mg) per reconstituted solution; i.e., it is essentially "sodium-free".
3. How to use Gonasi Kit
Gonasi Kit is a powder that must be dissolved with a liquid (solvent) before use; it is administered by injection under the skin (subcutaneously) or into a muscle (intramuscularly). The solution is obtained by combining the solvent with the powder and must be used immediately after preparation.
Follow the instructions for administration of the medicinal product indicated by your doctor. If in doubt, ask your doctor or pharmacist.
How to administer Gonasi Kit:
This medicinal product is administered by injection under your skin (subcutaneously) or into your muscle (intramuscularly).
Each vial is for single use only and the injection should be used as soon as it is prepared.
The recommended dose of this medicinal product is 5,000 IU or 10,000 IU to be administered 24-48 hours after optimal stimulation of follicular development.
After appropriate advice and training, your doctor may ask you to inject the medicinal product yourself.
In the case of the first time, your doctor should:
- have explained how to prepare the correct dose for injection
- have indicated how to prepare the injectable solution
- have indicated the areas where you can inject yourself
- have allowed you to practice the application of a subcutaneous injection on yourself.
Before injecting this medicinal product yourself, read the following instructions carefully.
How to prepare Gonasi Kit using 1 vial of powder
The solution must be prepared just before injection. Each vial is for single use only.
This medicinal product should only be reconstituted with the solvent supplied in the pack using the following step-by-step instructions:
Step 1
Wash your hands before preparing the solution. Use a clean surface for preparation. It is important that your hands and the materials you use are as clean as possible.
Step 2
Place the following materials on the clean surface:
- two alcohol-impregnated cotton balls (not supplied in the pack)
- a vial containing Gonasi Kit powder
- a solvent in a pre-filled syringe
- a long needle for reconstitution and for intramuscular injection.
- a short needle for subcutaneous injection.
Step 3
- Remove only the cap from the pre-filled syringe;
- Insert the needle for reconstitution (long needle) with its protective cap in place and check that the needle is properly placed, to avoid loss of solution. If solution is lost, try to fix the needle better with a slight rotation.
- Place the syringe carefully on the clean surface.
- Avoid touching the needle.
Step 4
- Remove the plastic closure cap from the Gonasi Kit vial by pushing it gently upwards.
- Clean the rubber stopper with an alcohol-impregnated cotton ball and wait for it to dry.
Step 5
- Take the syringe, remove the protective cap from the needle and push the needle through the center of the rubber stopper of the Gonasi Kit vial.
- Press the plunger down firmly and slowly inject the solvent into the vial with powder through the rubber stopper, to pour all the solution into the vial containing the powder.
- DO NOT SHAKE, gently roll the vial between your hands until the powder is completely dissolved, taking care not to form foam.
Step 6
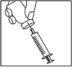
Once the powder is dissolved (which usually occurs immediately), slowly load the solution into the syringe as described below:
- With the needle still inserted, place the vial upside down.
- Make sure the tip of the needle is below the level of the liquid.
- Gently pull the plunger to load all the solution into the syringe.
- Check that the reconstituted solution is transparent and colorless.
Preparation of higher doses, using more than 1 vial of powder
If your doctor has recommended the highest dose of 10,000 IU, it can be obtained using two vials of powder with a pre-filled syringe of solvent.
When preparing 2 vials of the medicinal product, at the end of the previous step 3, load the reconstituted contents of the first vial into the syringe and slowly inject it into a second vial. Repeat steps 4-6 with the other vial.
The solution must be transparent and colorless.
Intramuscular injection of your medicinal product
For intramuscular injections, your doctor or nurse will prepare and then inject this medicinal product into the side of your thigh or buttock.
Subcutaneous injection of your medicinal product
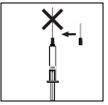 When the syringe contains the correct dose for you, place the protective cap on the long needle. Remove the long needle from the syringe and replace it with the short needle for subcutaneous injection with its protective cap in place. Please note that the needle must be properly placed and press the short needle firmly onto the syringe cylinder, then rotate it slightly to ensure it is completely screwed on, to create a firm seal to avoid loss of solution.
When the syringe contains the correct dose for you, place the protective cap on the long needle. Remove the long needle from the syringe and replace it with the short needle for subcutaneous injection with its protective cap in place. Please note that the needle must be properly placed and press the short needle firmly onto the syringe cylinder, then rotate it slightly to ensure it is completely screwed on, to create a firm seal to avoid loss of solution.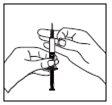 Remove the protective cap from the needle. Hold the syringe with the needle upwards and gently tap the side of the syringe to force any air bubbles to rise;
Remove the protective cap from the needle. Hold the syringe with the needle upwards and gently tap the side of the syringe to force any air bubbles to rise;- Press the plunger until a drop of liquid appears at the tip of the needle.
- DO NOT USE the solution if it contains particles or is cloudy.
The injection site:
 Your doctor or nurse will have already advised you on which area of your body to inject the medicinal product. The usual sites are the thigh or the lower abdominal wall (stomach area), below the navel.
Your doctor or nurse will have already advised you on which area of your body to inject the medicinal product. The usual sites are the thigh or the lower abdominal wall (stomach area), below the navel.- Clean the injection site with an alcohol-impregnated cotton ball.
- Pinch and firmly press the skin. With the other hand, insert the needle with a dart-like motion at an angle of 45° or 90°.
Injection of the solution:
- Inject under the skin, as indicated by your doctor.
- Do not inject directly into a vein.
- Press the plunger slowly and constantly, to correctly inject the solution and not damage the skin tissues.
Take all the time you need to inject the prescribed volume of solution.
Removal of the needle:
- Remove the syringe quickly and press on the injection site
- A gentle massage in the area – maintaining pressure – helps to disperse the medicinal product solution and alleviate any discomfort.
Dispose of all used material:
Once the injection is complete, all needles and empty syringes must be disposed of in a suitable container. The disposal of unused solution and all materials that have come into contact with it will be carried out in accordance with local regulations.
If you use more Gonasi Kit than you should:
The effects of an overdose of this medicinal product are unknown, however, it can be expected that Ovarian Hyperstimulation Syndrome (OHSS) may occur (see "Possible side effects"). If you use more of the medicinal product than you should, consult your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone: 91 562 04 20, indicating the medicinal product and the amount ingested.
If you forget to use Gonasi Kit:
If you forget to use the medicinal product, consult your doctor immediately.
If you stop treatment with Gonasi Kit:
Consult your doctor if you are considering not using this medicinal product.
If you have any other questions about the use of this medicinal product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
If you notice any of the following serious adverse effects, stop using Gonasi Kit and consult a doctor immediately, as you may need urgent medical treatment:
- mild or moderate ovarian hyperstimulation (ovarian hyperstimulation syndrome), which manifests as an increase in ovarian size, ovarian cysts, abdominal pain with vomiting and nausea (see also section 2 on "Ovarian Hyperstimulation Syndrome"). This is a frequent adverse effect (may affect up to 1 in 10 people).
- severe ovarian hyperstimulation (ovarian hyperstimulation syndrome) characterized by pain in the lower abdomen (pelvis), nausea, vomiting, weight gain, fluid accumulation in the abdomen (ascites) or in the chest (pleural effusion). This episode is uncommon (may affect up to 1 in 100 people).
- ovarian cyst rupture (a rare consequence of severe OHSS, may affect up to 1 in 1,000 people)
- formation of blood clots in blood vessels (thromboembolic episodes), as a complication of ovarian hyperstimulation syndrome. This episode is rare (may affect up to 1 in 1,000 people)
- severe generalized allergic reactions that may include: swelling of the face, eyes, lips, throat, or tongue, difficulty breathing, wheezing, skin rash, a rare adverse effect (may affect up to 1 in 1,000 people)
Other Adverse Effects
Frequent Adverse Effects:may affect up to 1 in 10 people
- Reaction at the injection site that may include redness, bruising, swelling, itching, or pain at the injection site
- swelling (edema)
- mood changes
- headache
- chest pain
Uncommon Adverse Effects:may affect up to 1 in 100 people
- restlessness (agitation), fatigue
Rare Adverse Effects:may affect up to 1 in 1,000 people
- swelling of the deep layers of the skin, often observed with hives (urticaria)
- generalized skin rashes
If you experience any of the rare side effects mentioned above, consult your doctor immediately, who will assess whether you should stop treatment with this medicine or go to the nearest hospital emergency department.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Gonasi Kit
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Keep the vial and the pre-filled syringe with solvent in the outer packaging to protect them from light.
Use immediately after preparing the solution.
Do not use this medicine after the expiration date that appears as "after CAD" on the outer packaging, the vial, and the pre-filled syringe with solvent. If the expiration date is expressed as month/year, the expiration date is the last day of the indicated month.
Do not use this medicine if you notice that the solution does not have a transparent appearance (turbid or with visible particles). The solution must be transparent and colorless after reconstitution.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE  point at the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
point at the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition ofGonasi Kit
The active ingredient is: human chorionic gonadotropin.
The excipients are:
- in the powder vial: lactose monohydrate
- in the pre-filled syringe with solvent (0.9% sodium chloride): water for injectable preparations, sodium chloride.
Each vial contains: 5,000 IU of human chorionic gonadotropin, produced from human urine.
Appearance ofthe Productand Package Contents
Gonasi Kit is presented as:
Powder in vial: white to almost white lyophilized powder
Solvent in pre-filled syringe (0.9% sodium chloride): clear and colorless solution
A single unit package contains:
A plastic tray that includes powder in a vial (type I glass), sealed with a rubber stopper and held in place with an easy-to-open cap.
1 ml of solvent in a pre-filled syringe (type I glass), 1 long needle for reconstitution and for intramuscular injection, and 1 short needle for subcutaneous injection.
A multiple package contains 10 plastic trays, as described above.
Only certain package sizes may be marketed.
Marketing Authorization Holder:
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia 2
26900 Lodi (Italy)
Manufacturer:
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italy
You can request more information about this medicine by contacting the local representative ofthe Marketing Authorization Holder:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Spain)
This medicine is authorized in the Member States of the European Economic Area with the following names: (The dose and pharmaceutical form are identical in all countries, only the trade name changes)
Denmark: Gonasi Set
Czech Republic: Zivafert
Spain: Gonasi Kit
Finland: Gonasi Set
France: GONADOTROPHINE CHORIONIQUE IBSA
Hungary: Zivafert Kit
Netherlands: Gonasi
Norway: Gonasi Set
Poland: Zivafert
Sweden: Gonasi Set
Slovak Republic: Gonasi Kit
Date of the last revision of this prospectus:January 2025
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GONASI KIT 5,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, 150 IU/0.25 ml (11 micrograms/0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for GONASI KIT 5,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about GONASI KIT 5,000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






