
REKOVELLE 36 micrograms/1.08 ml injectable solution in pre-filled pen

How to use REKOVELLE 36 micrograms/1.08 ml injectable solution in pre-filled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
REKOVELLE 36micrograms/1.08ml
injectable solution in pre-filled pen
folitropin delta
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What REKOVELLE is and what it is used for
- What you need to know before you use REKOVELLE
- How to use REKOVELLE
- Possible side effects
- Storage of REKOVELLE
- Contents of the pack and other information
1. What REKOVELLE is and what it is used for
REKOVELLE contains folitropin delta, a follicle-stimulating hormone that belongs to the family of hormones called gonadotropins. Gonadotropins are involved in reproduction and fertility.
REKOVELLE is used to treat female infertility and in women undergoing assisted reproduction techniques such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). REKOVELLE stimulates the ovaries to grow and develop multiple sacs (‘follicles’), from which eggs are obtained that are fertilized in the laboratory.
2. What you need to know before you use REKOVELLE
Before starting treatment with this medicine, a doctor must evaluate you and your partner to examine the possible causes of the infertility problem.
Do not use REKOVELLE
- if you are allergic to follicle-stimulating hormone or to any of the other ingredients of this medicine (listed in section 6)
- if you have a tumor in the uterus, ovaries, breasts, pituitary gland, or hypothalamus
- if you have enlarged ovaries or cysts not caused by polycystic ovary syndrome
- if you have vaginal bleeding of unknown cause
- if you have had early menopause
- if you have malformations of the sexual organs that make a normal pregnancy impossible
- if you have uterine fibroids that make a normal pregnancy impossible.
Warnings and precautions
Consult your doctor before using REKOVELLE
Ovarian Hyperstimulation Syndrome
Gonadotropins, such as this medicine, can cause Ovarian Hyperstimulation Syndrome. This occurs when your follicles develop excessively and become large cysts.
Consult your doctor if:
- you experience pain, discomfort, or swelling in the abdomen
- you experience nausea
- you experience vomiting
- you experience diarrhea
- you experience weight gain
- you have difficulty breathing
Your doctor may ask you to interrupt treatment with this medicine (see section 4).
If you follow the recommended dose and administration schedule, it is less likely that Ovarian Hyperstimulation Syndrome will occur.
Blood Clotting Problems (Thromboembolic Events)
The formation of blood clots in blood vessels (veins or arteries) is more likely in pregnant women. Infertility treatments can increase the risk of this happening, especially if you are overweight or you or a family member have a known blood clotting problem (thrombophilia). Consult your doctor if you think this applies to you.
Ovarian Torsion
There have been reports of ovarian torsion after assisted reproduction treatment. Ovarian torsion can cut off the blood flow to the ovaries.
Multiple Pregnancy and Birth Defects
When undergoing assisted reproduction treatment, the likelihood of having a multiple pregnancy (such as twins) is mainly related to the number of embryos transferred to your uterus, the quality of the embryos, and your age. Multiple pregnancy can lead to medical complications for you and your babies. Additionally, the risk of birth defects may be slightly higher when undergoing infertility treatment, which is thought to be due to the characteristics of the parents (such as age and sperm characteristics) and multiple pregnancy.
Pregnancy Loss
When undergoing assisted reproduction treatment, it is more likely that you will experience a miscarriage than with natural conception.
Ectopic Pregnancy
When undergoing assisted reproduction treatment, it is more likely that you will have an ectopic pregnancy than with natural conception. If you have a history of tubal disease, the risk of ectopic pregnancy is increased in your case.
Ovarian Tumors and Other Reproductive System Tumors
There have been reports of ovarian tumors and other reproductive system tumors in women who have undergone infertility treatment. It is not known whether treatment with fertility medicines increases the risk of these tumors in infertile women.
Other Medical Conditions
Before starting treatment with this medicine, tell your doctor if:
- another doctor has told you that pregnancy could be dangerous for you.
- you have liver or kidney disease
Children and Adolescents (under 18 years of age)
This medicine is not intended for use in children and adolescents.
Other Medicines and REKOVELLE
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and Breast-feeding
Do not use this medicine if you are pregnant or breast-feeding.
Driving and Using Machines
This medicine does not affect your ability to drive or use machines.
REKOVELLE contains sodium
This medicine contains less than 1 mmol of sodium chloride (23 mg) per dose, which is essentially ‘sodium-free’.
3. How to use REKOVELLE
Follow the instructions for administration of this medicine exactly as prescribed by your doctor.
In case of doubt, consult your doctor or pharmacist again.
The dose of REKOVELLE for the first treatment cycle will be calculated by your doctor using the level of anti-Müllerian hormone (AMH - a marker of how your ovaries will respond to stimulation with gonadotropins) in your blood and your body weight. Therefore, before starting treatment, the result of the AMH from a blood sample (taken in the last 12 months) must be available. Your body weight will also be measured before starting treatment. The dose of REKOVELLE is expressed in micrograms.
The dose of REKOVELLE is fixed throughout the treatment period, without adjustments to increase or decrease the daily dose. Your doctor will monitor the effect of treatment with REKOVELLE, and treatment will be discontinued when an adequate number of follicles have developed. In general, you will be given a single injection of a medicine called human chorionic gonadotropin (hCG) with a dose of 250 micrograms or 5,000 IU for the final development of the follicles.
If your body responds very weakly or very strongly to treatment, your doctor may decide to discontinue treatment with REKOVELLE. For the next treatment cycle, your doctor may give you a higher or lower daily dose of REKOVELLE than before.
How to administer the injections
The instructions for use of the pre-filled pen must be followed carefully. Do not use the pre-filled pen if the solution contains particles or does not appear clear.
The first injection of this medicine must be administered under the supervision of a doctor or nurse. Your doctor will decide if you can self-administer this medicine at home, but only after you have received adequate training.
This medicine is intended for subcutaneous injection, usually in the abdomen. The pre-filled pen can be used for multiple injections.
If you use more REKOVELLE than you should
The effects of using too much medicine are not known. It is possible that Ovarian Hyperstimulation Syndrome (OHSS) may occur, which is described in section 4.
If you forget to use REKOVELLE
Do not administer a double dose to make up for forgotten doses. Please contact your doctor or pharmacist as soon as you realize you have forgotten a dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects:
The hormones used in infertility treatment, such as this medicine, can cause high levels of activity in the ovaries (Ovarian Hyperstimulation Syndrome). Symptoms can include pain, discomfort, and swelling in the abdomen, nausea, vomiting, diarrhea, weight gain, or difficulty breathing. If you experience any of these symptoms, please contact your doctor immediately.
The risk of side effects is classified into the following categories:
Common (may affect up to 1 in 10 people):
- Headache
- Nausea
- Ovarian Hyperstimulation Syndrome (see above)
- Pelvic pain and discomfort, including that originating in the ovaries
- Fatigue
Uncommon (may affect up to 1 in 100 people):
- Mood changes
- Drowsiness / Dizziness
- Dizziness
- Diarrhea
- Vomiting
- Constipation
- Abdominal discomfort
- Vaginal bleeding
- Breast tenderness (including breast pain, breast swelling, breast sensitivity, and/or nipple pain)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of REKOVELLE
Keep out of the sight and reach of children.
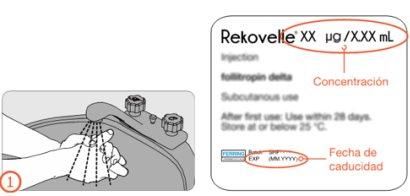
Do not use this medicine after the expiry date which is stated on the label of the pre-filled pen and the carton after ‘EXP’. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C - 8°C). Do not freeze.
Keep the pre-filled pen in the original package to protect it from light.
REKOVELLE can be stored at or below 25°C for up to 3 months, including the period after the first use. It must not be refrigerated again and must be discarded if not used after 3 months.
After the first use: 28 days when stored at or below 25°C.
At the end of the treatment, the unused product must be discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of REKOVELLE
- The active substance is folitropin delta.
Each pre-filled pen with a multi-dose cartridge contains 36 micrograms of folitropin delta in 1.08 milliliters of solution. One milliliter of solution contains 33.3 micrograms of folitropin delta per milliliter of solution.
- The other ingredients are phenol, polysorbate 20, L-methionine, sodium sulfate decahydrate, disodium phosphate dodecahydrate, concentrated phosphoric acid, sodium hydroxide, and water for injection.
Appearance of the Product and Container Contents.
REKOVELLE is a clear and colorless solution for injection in a pre-filled pen. It is available in packs of 1 pre-filled pen and 9 needles.
Marketing Authorization Holder
Ferring Pharmaceuticals A/S
Amager Strandvej 405
2770 Kastrup
Denmark
Manufacturer
Ferring GmbH
Wittland 11
D-24109 Kiel
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Ferring N.V. Tel/Tél: +32 53 72 92 00 | Lithuania CentralPharma Communications UAB Tel: +370 5 243 0444 |
Bulgaria Farmont Ltd. Tel: +359 2 807 5022 | Luxembourg/Luxemburg Ferring N.V. Belgique/Belgien Tel/Tél: +32 53 72 92 00 |
Czech Republic Ferring Pharmaceuticals CZ s.r.o. Tel: +420 234 701 333 | Hungary Ferring Magyarország Gyógyszerkereskedelmi Kft. Tel: +36 1 236 3800 |
Denmark Ferring Lægemidler A/S Tlf: +45 88 16 88 17 | Malta E.J. Busuttil Ltd. Tel: +356 21447184 |
Germany Ferring Arzneimittel GmbH Tel: +49 431 5852 0 | Netherlands Ferring B.V. Tel: +31 235680300 |
Estonia CentralPharma Communications OÜ Tel: +372 601 5540 | Norway Ferring Legemidler AS Tlf: +47 22 02 08 80 |
Greece Ferring Ελλάς ΜΕΠΕ Τηλ: +30 210 68 43 449 | Austria Ferring Arzneimittel Ges.m.b.H Tel: +43 1 60 8080 |
Spain Ferring S.A.U. Tel: +34 91 387 70 00 | Poland Ferring Pharmaceuticals Poland Sp. z o.o. Tel: +48 22 246 06 80 |
France Ferring S.A.S. Tél: +33 1 49 08 67 60 | Portugal Ferring Portuguesa – Produtos Farmacêuticos, Sociedade Unipessoal, Lda. Tel: +351 21 940 51 90 |
Croatia Clinres farmacija d.o.o. Tel: +385 1 2396 900 | Romania Ferring Pharmaceuticals Romania SRL Tel: +40 356 113 270 |
Ireland Ferring Ireland Ltd. Tel: +353 1 4637355 | Slovenia SALUS, Veletrgovina, d.o.o. Tel: +386 1 5899 100 |
Iceland Vistor hf. Sími: +354 535 70 00 | Slovakia Ferring Slovakia s.r.o. Tel: +421 2 54 416 010 |
Italy Ferring S.p.A. Tel: +39 02 640 00 11 | Finland Ferring Lääkkeet Oy Puh/Tel: +358 207 401 440 |
Cyprus A.Potamitis Medicare Ltd Τηλ: +357 22583333 | Sweden Ferring Läkemedel AB Tel: +46 40 691 69 00 |
Latvia CentralPharma Communications SIA Talr: +371 674 50497 | United Kingdom(Northern Ireland) Ferring Ireland Ltd Tel: +353 1 4637355 |
Date of Last Revision of this Leaflet.
Detailed and up-to-date information on this medicine is available on the European Medicines Agency (EMA) website: http://www.ema.europa.eu.
Instructions for Use
REKOVELLE Pre-filled Pen
folitropin delta
Your healthcare professional should teach you how to properly prepare and inject REKOVELLE before you inject it for the first time.
Do not attempt to inject it yourself until you have been trained by your healthcare professional in the correct way to administer the injections.
Read this entire manual before using the REKOVELLE pre-filled pen and each time you acquire a new pen. There may be new information. Follow these instructions carefully, even if you have used a similar injectable pen before. Incorrect use of the pen may result in an incorrect dose of the medicine.
Contact your healthcare professional (doctor, nurse, or pharmacist) if you have any questions about how to administer your REKOVELLE injection.
The REKOVELLE pre-filled pen is a disposable, dosing pen that can be used for the administration of more than 1 dose of REKOVELLE. The pen is available in 3 different concentrations:
- 12 micrograms/0.36 mL
- 36 micrograms/1.08 mL
- 72 micrograms/2.16 mL
REKOVELLE Pre-filled Pen and its Parts
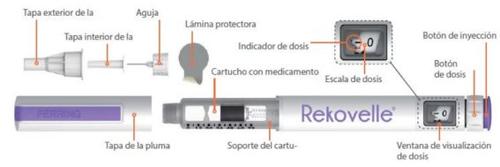
Instructions for Use – REKOVELLE Pre-filled Pen (folitropin delta)
Important Information
- The REKOVELLE pre-filled pen and needles are for single-person use and must not be shared with others.
- Use the pen only for the medical condition for which it was prescribed and as your healthcare professional has instructed.
- If you are blind or have poor vision and cannot read the dose scale on the pen, do not use this pen without assistance. Ask for help from a person with good vision and who is trained in how to use the pen.
- If you have any questions, contact your healthcare professional or the local representative of the marketing authorization holder (see the leaflet for contact information) before administering the REKOVELLE injection.
Information about your REKOVELLE Pre-filled Pen
The pen can be set for the administration of doses from 0.33 micrograms to 20 micrograms of REKOVELLE in marked increments of 0.33 micrograms. See “Examples of how to set a dose” on pages 20 to 211.
- The dose scale on the pen is numbered from 0 to 20 micrograms.
- Each number is separated by two lines, each line equivalent to an increment of 0.33 micrograms.
- When you turn the dose button clockwise, you will hear a click and feel resistance on the button for each increment to help you set the correct dose.
Cleaning
- The outside of your pen can be cleaned with a damp cloth, if necessary.
- Do not put the pen in water or any other liquid.
Storage
- Always keep the pen with the cap on and without a needle attached.
- Do not use the pen after the expiration date (EXP) printed on the pen label.
- Do not store the pen at extreme temperatures, direct sunlight, or cold conditions, such as a car or freezer.
- Keep the pen out of the reach of children and any person who has not been trained in the use of the pen.
Before Use:
- Store the pen in the refrigerator between 2 °C and 8 °C. Do not freeze.
- If stored outside the refrigerator (at a temperature equal to or below 25 °C), the pen can be stored for up to 3 months, including the usage period. Discard the pen if it has not been used after 3 months.
After First Use (Usage Period):
- The pen can be stored for up to 28 days at a temperature equal to or below 25°C. Do not freeze.
Items You Will Need to Administer Your REKOVELLE Injection
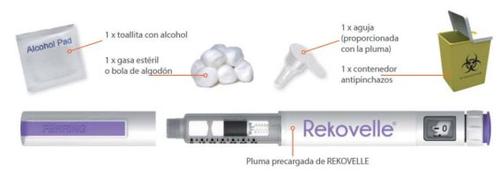
Before Use – (Step 1)
Step 1:
- Wash your hands.
- Check the pen for damage. Do not use the pen if it is damaged.
- Check the pen (cartridge) to ensure the medicine is clear and free of particles. Do not use a pen that contains particulate or cloudy medicine in the cartridge.
- Make sure you have the correct pen with the correct concentration.
- Check the expiration date on the pen label.
Attaching the Needle – (Steps 2 to 6)
Important:
- Always use a new needle for each injection.
- Use only the click-on needles for single use that are provided with the pen.
Step 2:
- Remove the pen cap.
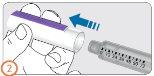
Step 3:
- Remove the needle protective cover.
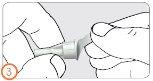
Step 4:
- Attach the needle.
- You will hear or feel a “click” when the needle is securely attached.
- You can also screw the needle on. When you feel slight resistance, it is securely attached.

Step 5:
- Remove the outer protective cap of the needle.
- Do not discard the outer needle cap. You will need it to dispose of the needle after the injection.
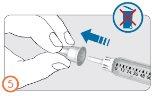
Step 6:
- Remove the inner needle cap and discard it.
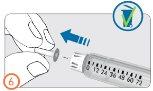
Preparation – (Steps 7 to 9)
- Before using the pen for the first time, you must remove air bubbles from the cartridge (priming) to receive the correct dose of medicine.
- You should only prepare the pen the first time you use it.
- Perform steps 7 to 9 even if you do not see air bubbles.
- If the pen has already been used, go directly to step 10.
Step 7:
- Turn the dose button clockwise until the drop symbol is aligned with the dose indicator.
- If you set the wrong preparation dose, it can be corrected both upwards and downwards without loss of medicine by turning the dose button in either direction until the drop symbol is aligned with the dose indicator.
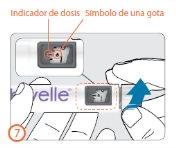
Step 8:
- Hold the pen with the needle pointing upwards.
- Tap the cartridge holder gently with your finger to make any air bubbles in the cartridge rise to the top of the cartridge.
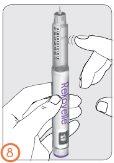
Step 9:
- With the needle still pointing upwards (away from your face), press the injection button until you see the number ‘0’ in line with the dose indicator.
- Check that a drop of liquid appears at the tip of the needle.
- If the drop(s) do not appear, repeat steps 7 to 9 (Preparation) until a drop appears.
- If a drop does not appear after 5 attempts, remove the needle (see step 13), and attach a new needle (see steps 3 to 6), and repeat the preparation (see steps 7 to 9).
(If you still do not see a drop after using a new needle, try with a new pen.)
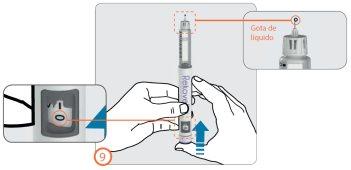
Setting the Dose – (Step 10)
See “Examples of how to set a dose” on pages 20 to 211.
Step 10:
- Turn the dose button clockwise until the prescribed dose is aligned with the dose indicator in the dose viewing window.
- The dose can be corrected both upwards and downwards without loss of medicine by turning the dose button in either direction until the correct dose is aligned with the dose indicator.
- Do not press the injection button when setting the dose to avoid loss of medicine.

Dose Division:
- You may need more than one pen to complete the dose that has been prescribed for you.
- If you are unable to set your complete dose, this means that there is not enough medicine left in the pen. You will need to administer the dose divided into two injections or discard your pen and use a new one for your injection.
See “Administering a Divided Dose of REKOVELLE” on pages 22 to 231 for examples of how to calculate and record your divided dose.
Injecting the Dose – (Steps 11 to 12)
Important:
- Do not use the pen if the medicine contains particles or is cloudy.
- Read steps 11 and 12 on pages 14 to 151 before administering your injection.
- This medicine must be administered by injection just under the skin (subcutaneously) in the stomach area (abdomen).
- Use a new injection site for each injection to minimize the risk of skin reactions such as redness and irritation.
- Do not inject into an area that is irritated (sensitive), bruised, red, hard, scarred, or has stretch marks.
Steps 11 and 12:
- Clean the skin where you will inject with an alcohol swab. Do not touch this area again before administering the injection.
- Hold the pen so that the dose viewing window is visible during the injection.
- Pinch your skin and insert the needle directly as your healthcare professional has taught you. Do not press the injection button yet.
- After inserting the needle, place your thumb on the injection button.
- Press the injection button all the way down and hold it.
- Continue to hold the injection button and when you see the number ‘0’ in line with the dose indicator, wait 5 seconds (counting slowly to 5). This will ensure you receive your complete dose.
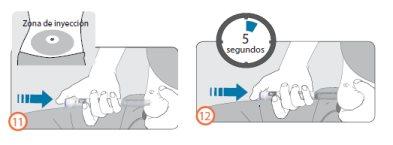
- After pressing the injection button for 5 seconds, release it. Then, slowly remove the needle from the injection site, pulling it straight out of the skin.
- If blood appears at the injection site, press lightly on the area with a gauze or cotton ball.
Note:
- Do not tilt the pen during injection or during needle removal.
- Tilting the pen can cause the needle to bend or break.
- If a broken needle remains stuck in the body or under the skin, seek immediate medical help.
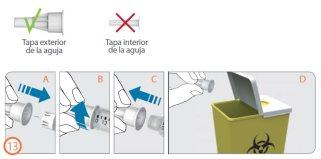
Disposing of the Needle – (Step 13)
Step 13:
- Carefully replace the outer needle cap with a firm push (A).
- Unscrew the needle in a counterclockwise direction to remove it from the pen (B+C).
- Carefully dispose of the used needle (D).
- See “Disposal” on page 181.
Note:
- Always remove the needle after each use. Needles are for single use only.
- Do not store the pen with the needle attached.
Replacing the Cap on the Pen – (Step 14)
Step 14:
- Firmly replace the pen cap to protect it between injections.
Note:
- The pen cap will not fit if a needle is attached.
- If you are going to administer a divided dose in two injections, discard the pen only when it is empty.
- If you are going to use a new pen to administer the complete dose that has been prescribed for you instead of administering a divided dose in two injections, discard the pen when there is not enough medicine left for a complete dose.
- Keep the pen cap on when not in use.
Disposal
Needles:
Place used needles in a sharps container, such as a container for sharp objects, immediately after use. Do not discard the used container with your household waste.
If you do not have a sharps container, you can use a household container that has the following characteristics:
Following characteristics:
- It should be made of solid and resistant plastic,
- it can be closed with a tight and puncture-resistant cap, without the puncture material being able to escape to the outside,
- it can remain upright and stable during use,
- it is leak-proof, and
- it is correctly labeled to warn that it contains hazardous waste.
REKOVELLE pre-filled pens:
- Discard used pens according to local waste disposal legislation.
Examples of how to dial a dose
Examples of how to dial a dose using your REKOVELLE pre-filled pen
The following table shows examples of prescribed doses, how to dial the examples of prescribed doses, and what the dose display window looks like for the prescribed doses.
Examples of prescribed doses (in micrograms) | Dose to be dialed on the pen | Dose display window for the examples of prescribed dose |
0.33 | 0 and 1 line (dial up to 0 plus 1 click) |
|
0.66 (preparation dose) | 0 and 2 lines (dial up to 0 plus 2 clicks) |
|
2.33 | 2 and 1 line (dial up to 2 plus 1 click) |
|
11.00 | 11 (dial up to 11) |
|
12.33 | 12 and 1 line (dial up to 12 plus 1 click) |
|
18.66 | 18 and 2 lines (dial up to 18 plus 2 clicks) |
|
20.00 | 20 (dial up to 20) |
|
Administration of a split dose of REKOVELLE
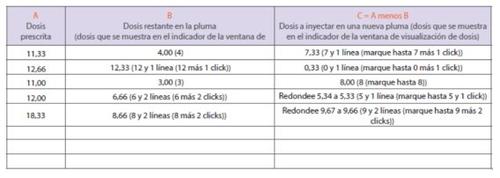
If you are unable to dial the complete prescribed dose on your pen, this means that there is not enough medication left in the pen to administer the complete dose. You will need to administer part of your prescribed dose using the pen you are using, and the rest of the dose using a new pen (split dose injection) or you will need to discard the pen you are using and use a new pen to administer the complete prescribed dose in a single injection. If you decide to administer the split dose in two injections, follow these instructions and write down the amount of medication to be administered using the split dose diary on page 231.
- Column A shows an example of a prescribed dose. Write down the dose that has been prescribed for you in column A.
- Column B shows an example of the dose left in the pen (this is the same as the dose you are able to dial).
- Write down the dose left in your pen in column B. Administer the injection using the remaining amount of medication left in your pen.
- Prepare a new pen (steps 1 to 9).
- Calculate and write down the remaining dose to be injected in column C, to do this you must subtract the number in column B from the number in column A. Use a calculator to check that you have done the operation correctly, if necessary.
- See “Examples of how to dial a dose” on pages 20 to 211 if necessary.
- Doses should be rounded to the nearest increment, X.00, X.33, or X.66 micrograms. For example, if the number in column C is 5.34, round your dose to 5.33. If the number in column C is 9.67, round your dose to 9.66.
- Contact your healthcare professional if you have any doubts about how to calculate your split dose.
- Inject the remaining dose of medication (the number in column C) using a new pen to complete the prescribed dose.
Split dose diary
Frequently asked questions
- Is the preparation step necessary before each injection?
- No. Preparation should only be done before the administration of the first injection with a new pen.
- How can I know if the injection is complete?
- The injection button has been pressed firmly to the end until it has stopped.
- The number ‘0’ is in line with the dose indicator.
- You have counted slowly to 5 while holding the injection button and the needle is still injected in the skin.
- Why should I count to 5 while holding the injection button?
- Holding the injection button for 5 seconds allows the complete dose to be injected and absorbed under your skin.
- What happens if the dose button cannot be turned to the prescribed dose?
- It is possible that the pen cartridge does not have enough medication to deliver the prescribed dose.
- The pen does not allow you to dial a dose greater than what is left in the cartridge.
- You can inject the amount of medication left in the pen and complete the prescribed dose with a new pen (split dose) or use a new pen to administer the complete prescribed dose.
Precautions
- Do not use a pen that has been dropped or hit against hard surfaces.
- If it is not easy to press the injection button, do not use force. Change the needle. If the injection button continues to be difficult to press after changing the needle, use a new pen.
- Do not attempt to repair a damaged pen. If a pen is damaged, contact your healthcare professional or local representative of the marketing authorization holder (see the package leaflet for contact information).
Additional information
Needles
Needles are provided with the pen. If you need additional needles, contact your healthcare professional. Use only the needles that come with the REKOVELLE pre-filled pen or the needles that your healthcare professional prescribes for you.
Contact
If you have any questions or problems related to the pen, contact your healthcare professional or local representative of the marketing authorization holder (see the package leaflet for contact information).
- The page numbers refer to the printed Instructions for Use manual and not to the page numbers of this document.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REKOVELLE 36 micrograms/1.08 ml injectable solution in pre-filled penDosage form: INJECTABLE, 12 micrograms/0.36 mlActive substance: follitropin deltaManufacturer: Ferring Pharmaceuticals A/SPrescription requiredDosage form: INJECTABLE, 72 µgActive substance: follitropin deltaManufacturer: Ferring Pharmaceuticals A/SPrescription requiredDosage form: INJECTABLE, 150 IU/ 0.25 ml (11 micrograms/ 0.25 ml)Active substance: follitropin alfaManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for REKOVELLE 36 micrograms/1.08 ml injectable solution in pre-filled pen
Discuss questions about REKOVELLE 36 micrograms/1.08 ml injectable solution in pre-filled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













