
Vizimaco
Ask a doctor about a prescription for Vizimaco

How to use Vizimaco
Package Leaflet: Information for the User
Vizimaco, 0.3 mg/ml + 5 mg/ml, eye drops, solution
Bimatoprost + Timolol
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- This leaflet should be kept, so that it can be read again if necessary.
- In case of any doubts, the doctor, pharmacist, or nurse should be consulted.
- This medicine has been prescribed specifically for the patient. It should not be given to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet:
- 1. What is Vizimaco and what is it used for
- 2. Important information before using Vizimaco
- 3. How to use Vizimaco
- 4. Possible side effects
- 5. How to store Vizimaco
- 6. Contents of the pack and other information
1. What is Vizimaco and what is it used for
Vizimaco contains two active substances (bimatoprost and timolol) that lower the pressure in the eye.
Bimatoprost belongs to a group of medicines called prostamides, prostaglandin analogs. Timolol belongs
to a group of medicines called beta-adrenergic blockers.
The eye contains a clear, watery fluid that nourishes the inside of the eye. The fluid is constantly drained
from the eye and replaced by new fluid. If the fluid cannot be drained quickly enough, the pressure in the eye increases, which can eventually lead to vision damage (causing a disease called glaucoma). The action of Vizimaco is to reduce the production of fluid and increase the amount of drained fluid. This leads to a decrease in pressure inside the eye.
Vizimaco eye drops are used to treat high pressure in the eye in adults, including the elderly. High pressure can lead to glaucoma. The doctor will prescribe Vizimaco when the action of other eye drops containing beta-adrenergic blockers or prostaglandin analogs is insufficient.
This medicine does not contain preservatives.
2. Important information before using Vizimaco
When not to use Vizimaco eye drops, solution
- if the patient is allergic to bimatoprost, timolol, beta-adrenergic blockers, or any of the other ingredients of this medicine (listed in section 6);
- if the patient has had or has a respiratory disease, such as asthma and/or severe chronic obstructive pulmonary disease (a lung disease that can cause wheezing, difficulty breathing, and prolonged coughing) or other types of breathing problems;
- if the patient has heart disease, such as slow heart rate, heart block, or
heart failure.
Warnings and precautions
Before starting treatment with Vizimaco, the patient should discuss with their doctor if they currently have or have had any of the following conditions:
- coronary heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, low blood pressure,
- heart rhythm disturbances, such as slow heart rate,
- breathing disorders, asthma, or chronic obstructive pulmonary disease,
- circulatory disorders, such as Raynaud's disease or Raynaud's syndrome,
- hyperthyroidism, as timolol may mask the signs and symptoms of thyroid disease,
- diabetes, as timolol may mask the signs and symptoms of low blood sugar,
- severe allergic reactions,
- liver or kidney disease,
- eye surface diseases,
- detachment of one of the layers of the eye after surgery to lower eye pressure,
- known risk factors for macular edema (swelling of the retina in the eye leading to vision impairment), such as cataract surgery.
If the patient has a history of hypersensitivity to silver, they should not use this medicine.
Before anesthesia for surgery, the patient should tell their doctor about using Vizimaco, as timolol may affect the action of some anesthetics.
During treatment with Vizimaco, it may cause loss of fat tissue around the eye, which can lead to deepening of the eyelid fold, sunken eyes, drooping eyelids (ptosis), skin tightening around the eye (dermatochalasis), and increased visibility of the white part of the eye (scleral show). These changes are usually mild, but if they are pronounced, they may affect the field of vision. The changes may disappear after discontinuation of Vizimaco treatment.
Vizimaco may also cause darkening and excessive growth of eyelashes, and may also cause darkening of the skin around the eyelid. It may also darken the color of the iris. These changes may be permanent. The changes may be more noticeable if only one eye is treated. If Vizimaco comes into contact with the skin surface, it may cause hair growth.
Children and adolescents
Vizimaco should not be used in children and adolescents under 18 years of age.
Vizimaco and other medicines
Other medicines may affect the action of Vizimaco, and Vizimaco may affect the action of other medicines, including other eye drops used to treat glaucoma. The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
The patient should tell their doctor about taking or planning to take:
- medicines that lower blood pressure,
- medicines used to treat heart disease,
- medicines used to treat diabetes,
- quinidine (a medicine used to treat heart disease or certain types of malaria)
- medicines used to treat depression (fluoxetine and paroxetine).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. Vizimaco should not be used during pregnancy, unless the doctor recommends otherwise.
Vizimaco should not be used during breastfeeding. Timolol may pass into breast milk. Before taking any medicine during breastfeeding, the patient should consult their doctor.
Driving and using machines
Vizimaco may cause blurred vision in some patients. The patient should not drive vehicles or operate machines until the symptoms have resolved.
Vizimaco contains disodium phosphate anhydrous
The medicine contains 0.0285 mg of phosphates in each drop, which corresponds to 0.95 mg/ml.
In patients with severe damage to the clear, front part of the eye (cornea), phosphates may rarely cause corneal calcification during treatment due to calcium deposition.
3. How to use Vizimaco
This medicine should always be used exactly as the doctor or pharmacist has told the patient. In case of doubt, the patient should consult their doctor or pharmacist.
The recommended dose is one drop into each eye that needs treatment, once a day, in the morning or evening. The medicine should be used every day at the same time.
The patient should not touch the eye or the surface around the eye with the tip of the multidose container. This may cause eye damage. Eye drops, solution may become contaminated with bacteria, causing eye infections, leading to serious eye damage, or even vision loss.
To avoid possible contamination of the multidose container, the patient should avoid contact between the tip of the container and any surface.
Method of administration
Before instilling the eye drops:
- The patient should wash their hands before opening the bottle.
- The patient should not use the medicine if the seal on the neck of the bottle is broken before the first use.
- If the patient is using the medicine for the first time, they should check the use of the bottle by pressing it slowly and releasing one drop into the air, away from the eye.
- If the patient is sure they can release one drop at a time, they should take the most comfortable position to instill the drop into the eye (they can sit, lie on their back, or stand in front of a mirror).
Instillation of the eye drops
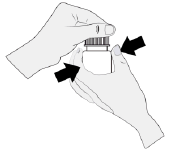
- 1. Hold the bottle directly under the cap and open it by twisting the cap. Do not touch the tip of the dropper to avoid contaminating the solution.
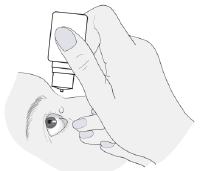
- 2. Tilt the head back and hold the bottle over the eye.
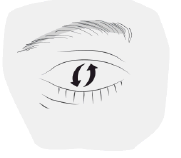
- 3. Pull the lower eyelid down and look up. Gently squeeze the middle part of the bottle and release one drop into the eye. Remember that it may take a few seconds from squeezing the bottle to the drop coming out. Do not squeeze the bottle too hard. If the drop does not get into the eye, try again. Wipe away any excess drops that spill onto the cheek.
In case of doubts about using the medicine, the patient should consult their doctor, pharmacist, or nurse.
- 4. To spread the medicine over the surface of the eye, blink several times.
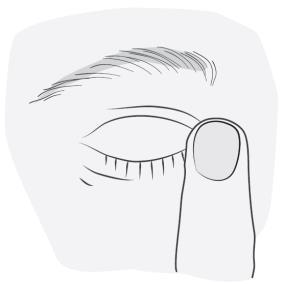
- 5. Without opening the eye, press the corner of the closed eye (near the nose) with a finger and hold for 2 minutes. This will prevent Vizimaco from entering the body.
- 6. If the doctor has instructed, repeat steps 2-5 to administer the medicine to the other eye. Sometimes treatment is only required for one eye; the doctor will inform the patient which eye to treat.
- 7. After using the medicine and before replacing the cap, shake the bottle once downwards without touching the tip, to remove any remaining medicine that may be on it. This is necessary for the correct dosing of subsequent drops.
- 8. After using all the doses, a small amount of solution will remain in the Vizimaco bottle. The patient should not worry about this, as the packaging contains an excess of Vizimaco, and the patient will receive the full amount of Vizimaco prescribed by the doctor. After completing the treatment cycle, the patient should not use the medicine that is still in the bottle. The drops are suitable for use for 28 days after first opening the bottle.
If Vizimaco is used with other eye medicines, the patient should wait at least 5 minutes between instilling Vizimaco and administering another medicine. Eye ointment or gel should be applied last.
Using more than the recommended dose of Vizimaco
In case of using more than the recommended dose of Vizimaco, it is unlikely to cause serious harm. The next dose should be taken at the usual time.
In case of doubts, the patient should contact their doctor or pharmacist.
Missing a dose of Vizimaco
If a dose of Vizimaco is missed, the patient should take one drop as soon as they remember, and then continue using the medicine as planned. The patient should not take a double dose to make up for the missed dose.
Stopping treatment with Vizimaco
To work properly, Vizimaco should be used every day.
In case of any further doubts about using this medicine, the patient should consult their doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Vizimaco can cause side effects, although not everybody gets them.
Generally, the patient can continue using the eye drops if the side effects are not severe.
In case of doubts, the patient should consult their doctor or pharmacist. The patient should not stop using Vizimaco without consulting their doctor.
The following side effects may occur during treatment with Vizimaco:
Very common side effects(may affect more than 1 in 10 people)
Related to the eye
- redness
Common side effects(may affect up to 1 in 10 people)
Related to the eye
- burning sensation
- itching
- stinging
- conjunctival hyperemia (redness of the clear membrane covering the eye)
- sensitivity to light
- eye pain
- eye discharge
- dry eye
- feeling of something in the eye
- small injuries on the surface of the eye (with or without inflammation)
- blurred vision
- redness and itching of the eyelids
- hair growth around the eye
- darkening of the eyelids
- darker skin tone around the eyes
- lengthening of eyelashes
- eye irritation
- excessive tearing
- swelling of the eyelids
- limited vision
Related to other parts of the body
- runny nose
- headache
Uncommon side effects(may affect up to 1 in 100 people)
Related to the eye
- abnormal sensations in the eye
- uveitis (inflammation of the uvea, the middle layer of the eye)
- conjunctival edema (swelling of the clear membrane covering the eye)
- eyelid pain
- eye fatigue
- ingrown eyelashes
- darkening of the iris
- eyelid retraction (the eyelid is pulled back)
- darkening of the eyelashes
Related to other parts of the body
- shortness of breath
Side effects with unknown frequency(cannot be estimated from the available data)
Related to the eye
- cystoid macular edema (swelling of the retina in the eye leading to vision impairment)
- eye edema
- blurred vision
- eye discomfort
Related to other parts of the body
- breathing difficulties / wheezing
- symptoms of allergic reactions (swelling, redness of the eye, and skin rash)
- taste disturbances
- dizziness
- slow heart rate
- high blood pressure
- sleep disturbances, nightmares
- asthma
- hair loss
- skin color changes (around the eye)
- fatigue
Additional side effects have been observed in patients using eye drops containing timolol or bimatoprost, and may also occur with Vizimaco.
Like other ophthalmic medications containing timolol, it may be absorbed into the systemic circulation. This may lead to similar side effects as seen with intravenous and/or oral administration of beta-adrenergic blockers. The frequency of systemic side effects after topical ophthalmic administration is lower than after oral or intravenous administration.
The following side effects have been reported with bimatoprost and timolol in the treatment of eye diseases:
- severe allergic reactions with swelling and difficulty breathing, which can be life-threatening
- low blood sugar
- depression, memory loss, hallucinations
- fainting, stroke, reduced blood flow to the brain, worsening of myasthenia (increased muscle weakness), tingling sensation
- reduced sensitivity of the eye surface, double vision, drooping eyelid, detachment of one of the layers of the eye after surgery to lower eye pressure, inflammation of the eye surface, bleeding in the back of the eye (retinal hemorrhage), eye inflammation, frequent blinking
- very common: loss of fat tissue around the eye, which can lead to deepening of the eyelid fold, sunken eyes, drooping eyelids (ptosis), skin tightening around the eye (dermatochalasis), and increased visibility of the white part of the eye (scleral show)
- heart failure, irregular heartbeat or heart arrest, slow or fast heart rate, fluid accumulation (mainly water) in the body, chest pain
- low blood pressure, swollen or cold hands, feet, and limbs due to narrowed blood vessels
- cough, worsening of asthma, worsening of chronic obstructive pulmonary disease (COPD)
- diarrhea, stomach pain, nausea and vomiting, indigestion, dry mouth
- red, flaky skin changes, skin rash
- muscle pain
- reduced sex drive, sexual disturbances
- weakness
- increased liver enzyme levels in blood tests
Other side effects reported with eye drops containing phosphates
In very rare cases, in some patients with severe damage to the clear, front part of the eye (cornea), phosphates may cause corneal calcification during treatment due to calcium deposition.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02 222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Vizimaco
The medicine should be stored out of sight and reach of children.
Vizimaco should not be used after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of the month.
This medicine does not require special storage conditions.
From a microbiological point of view, the medicine can be stored for a maximum of 28 days after first opening the bottle. There are no special storage conditions. The user is responsible for the storage conditions and shelf life of the medicine after opening.
The medicine should not be used if the seal on the neck of the bottle is broken before the first use.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Vizimaco contains
- The active substances of Vizimaco are bimatoprost 0.3 mg/ml and timolol 5 mg/ml (equivalent to 6.8 mg/ml timolol maleate).
- The other ingredients are: sodium chloride, disodium phosphate anhydrous, citric acid monohydrate, sodium hydroxide, and/or hydrochloric acid (to adjust the pH), water for injections.
What Vizimaco looks like and contents of the pack
Vizimaco is a 3 ml clear, colorless, aqueous solution, practically free from particles, which is supplied in a 5 ml white, opaque bottle with LDPE, with a Novelia system consisting of a dropper (with HDPE and silicone) and a cap with HDPE. The whole is placed in a cardboard box.
The following pack sizes are available: 1 or 3 bottles containing 3 ml of solution, in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
[email protected]
Manufacturer
EXCELVISION
27 st. La Lombardière
Zl La Lombardière
07100 Annonay
France
Pharmathen SA
6 Dervenakion str.
15351 Pallini Attiki
Greece
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterExcelvision Pharmathen S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VizimacoDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Vizimaco in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vizimaco in Spain
Alternative to Vizimaco in Ukraine
Online doctors for Vizimaco
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vizimaco – subject to medical assessment and local rules.














