
Akistan Duo
Ask a doctor about a prescription for Akistan Duo

How to use Akistan Duo
Leaflet accompanying the packaging: patient information
Akistan DUO, 50 micrograms/ml + 5 mg/ml, eye drops, solution
Latanoprost + Timolol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Akistan DUO and what is it used for
- 2. Important information before using Akistan DUO
- 3. How to use Akistan DUO
- 4. Possible side effects
- 5. How to store Akistan DUO
- 6. Contents of the packaging and other information
1. What is Akistan DUO and what is it used for
Akistan DUO contains two active substances: latanoprost and timolol. Latanoprost belongs to a group of medicines called prostaglandin analogues. Timolol belongs to a group of medicines called beta-adrenergic blockers.
Latanoprost increases the outflow of fluid from the eye into the bloodstream. Timolol reduces the amount of fluid produced in the eye.
Akistan DUO is used to reduce intraocular pressure in patients with open-angle glaucoma or increased intraocular pressure. Both conditions are associated with increased intraocular pressure and can affect vision.
The doctor will usually recommend using Akistan DUO if the action of other medicines has not been sufficient.
2. Important information before using Akistan DUO
Akistan DUO can be used in adults, including the elderly, but it is not recommended for use in children under 18 years of age.
When not to use Akistan DUO
Warnings and precautions
Before starting treatment with Akistan DUO, the patient should inform their doctor or pharmacist if they have or have had:
- coronary heart disease (symptoms may include chest pain or tightness, shortness of breath, or suffocation), heart failure, low blood pressure,
- arrhythmias, such as slow heart rate,
- breathing difficulties, asthma, or chronic obstructive pulmonary disease,
- diseases related to poor blood circulation (such as Raynaud's disease or Raynaud's syndrome),
- diabetes, as timolol may mask signs of low blood sugar,
- hyperthyroidism, as timolol may mask signs and symptoms,
- planned eye surgery (including cataract surgery) or if it has been performed in the past,
- eye problems (such as eye pain, eye irritation, eye inflammation, or blurred vision),
- dry eyes,
- if the patient uses contact lenses. Akistan DUO can still be used, but the patient should follow the instructions for using contact lenses in section 3,
- if the patient has variant angina (a type of angina known as Prinzmetal's angina),
- if the patient has had severe allergic reactions that usually require hospital treatment,
- if the patient has had a viral eye infection caused by the herpes simplex virus (HSV).
Before surgery, the patient should inform their doctor about using Akistan DUO, as timolol may affect the action of certain medicines used during anesthesia.
Akistan DUO and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, including eye drops and medicines that are available without a prescription.
Akistan DUO may interact with other medicines, including eye drops used to treat glaucoma. The patient should inform their doctor about using medicines that lower blood pressure, heart medicines, and medicines used to treat diabetes.
In particular, the patient should inform their doctor or pharmacist if they are using a medicine that belongs to any of the following groups:
- prostaglandins, analogues, or derivatives of prostaglandins,
- beta-adrenergic blockers,
- epinephrine,
- blood pressure-lowering medicines, such as oral calcium channel blockers, guanethidine, anti-arrhythmic medicines, cardiac glycosides, or parasympathomimetics,
- quinidine (used to treat heart disorders and certain types of malaria),
- antidepressants, such as fluoxetine, paroxetine.
Using Akistan DUO with food and drink
Consuming standard meals, food, or drinks does not affect when or how to use Akistan DUO.
Pregnancy, breastfeeding, and fertility
Pregnancy
Akistan DUO should not be used during pregnancy unless the doctor decides it is necessary.
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
Breastfeeding
Akistan DUO should not be used during breastfeeding. Akistan DUO may pass into breast milk. Before using any medicine during breastfeeding, the patient should consult their doctor.
Fertility
In animal studies, no effect of latanoprost and timolol on fertility in males or females has been found.
Driving and using machines
After using Akistan DUO, temporary blurred vision may occur. If the patient experiences this type of discomfort, they should not drive or operate machines until it resolves.
Akistan DUO contains benzalkonium chloride and phosphate buffer
The medicine contains 0.2 mg of benzalkonium chloride per milliliter, which corresponds to 0.006 mg per drop.
Benzalkonium chloride may be absorbed by soft contact lenses and may change the color of contact lenses. The patient should remove their contact lenses before using this medicine and put them back on after 15 minutes. Benzalkonium chloride may also cause eye irritation, especially if the patient has dry eyes or corneal disorders (the transparent layer on the front of the eye). If the patient experiences abnormal sensations in the eye, stinging, or eye pain after using this medicine, they should talk to their doctor.
Instructions for using contact lenses are provided in section 3.
This medicine contains 6.33 mg of phosphates per milliliter, which corresponds to 0.2 mg per drop. In patients with severe damage to the transparent, front part of the eye (cornea), phosphates may rarely cause corneal clouding due to calcium deposition during treatment.
3. How to use Akistan DUO
This medicine should always be used as directed by the doctor or pharmacist. If the patient has any doubts, they should consult their doctor or pharmacist.
The recommended dose for adult patients (including the elderly) is 1 drop into the affected eye(s) once a day.
Akistan DUO should not be used more than once a day, as this may reduce the effectiveness of the treatment.
Akistan DUO should be used as directed by the doctor, who will decide when to stop the treatment.
If the patient is using Akistan DUO, the doctor may recommend additional heart and circulatory system tests.
Using contact lenses
If the patient uses contact lenses, they should remove them before using Akistan DUO. After using Akistan DUO, the patient should wait 15 minutes before putting their contact lenses back on.
Instructions for use
| Fig. 1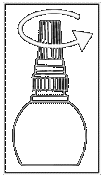 |
| Fig. 2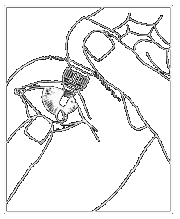 |
| Fig. 3 |
- 7. If the doctor has instructed, repeat the procedure for the second eye.
- 8. After use, replace the cap.
Using Akistan DUO with other eye drops
The patient should wait at least 5 minutes between using Akistan DUO and other eye drops.
Using more than the recommended dose of Akistan DUO
If more drops are administered than recommended, eye irritation, tearing, and eye redness may occur. These symptoms should resolve, but if the patient is concerned, they should consult their doctor for advice.
Accidental ingestion of Akistan DUO
In case of accidental ingestion of Akistan DUO, the patient should consult their doctor. If a large amount of eye drops is swallowed, the patient may experience discomfort, stomach pain, fatigue, facial flushing, and dizziness.
Missing a dose of Akistan DUO
If a dose is missed, the patient should take the next dose at the scheduled time. The patient should not take a double dose to make up for the missed dose. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Akistan DUO can cause side effects, although not everybody gets them.
Eye drops can usually be used until the side effects are not serious. If the patient has any doubts, they should consult their doctor or pharmacist. The patient should not stop using Akistan DUO without consulting their doctor.
The following are known side effects that have occurred with Akistan DUO.
The most important side effect is the possibility of gradual, permanent change in eye color. Akistan DUO may cause serious changes in heart function. The patient should inform their doctor if they notice any changes related to normal heart function or heart function and inform them about using Akistan DUO.
Known side effects that have occurred with Akistan DUO:
Very common (may affect more than 1 in 10 people):
- Gradual change in eye color associated with an increase in the amount of brown pigment in the colored part of the eye called the iris. In patients whose iris color is a mix of colors (such as blue-brown, gray-brown, yellow-brown, or green-brown), these changes can be noticed much more often than in people with eyes of a single color (blue, gray, green, or brown). The change in eye color may develop over several years. The change in eye color may be permanent and more noticeable if Akistan DUO is administered only to one eye. It seems that the change in eye color is harmless. After stopping Akistan DUO, the change in eye color does not progress.
Common (may affect up to 1 in 10 people):
- Eye irritation (including feelings of burning, grittiness, itching, stinging, or the sensation of a foreign body in the eye) and eye pain.
Uncommon (may affect up to 1 in 100 people):
- Headache
- Eye redness, eye infection (conjunctivitis), blurred vision, tearing, eyelid inflammation, eye irritation, or damage to the eye surface
- Skin rash or itching (pruritus)
- Nausea
- Vomiting
Frequency not known (frequency cannot be estimated from the available data):
- Hallucinations
Other side effects
Like other eye medicines, Akistan DUO (latanoprost and timolol) is absorbed into the bloodstream. The frequency of side effects with eye drops is lower than with oral or intravenous medicines.
The following are side effects that, although they have not occurred with Akistan DUO, have occurred with the individual components of this medicine (latanoprost and timolol) and may also occur with Akistan DUO. These include side effects observed during the use of beta-adrenergic blockers (e.g., timolol) in the treatment of eye diseases.
- Development of viral eye inflammation caused by the herpes simplex virus (HSV).
- General allergic reactions, including angioedema (swelling under the skin that can occur on the face, limbs, and can make it difficult to breathe), which can cause difficulty swallowing and breathing, urticaria, or itchy rash, localized and generalized rash, itching, severe life-threatening allergic reactions.
- Low blood sugar.
- Dizziness.
- Difficulty sleeping (insomnia), depression, nightmares, memory loss.
- Fainting, stroke, decreased blood flow to the brain, worsening of myasthenia symptoms (muscle weakness and pain in patients with this disease), feeling of tingling or numbness, and headache.
- Edema of the back of the eye (macular edema), formation of fluid-filled spaces in the iris (iris cysts), sensitivity to light (photophobia), changes in the eye socket and eyelids resulting in deepening of the eyelid fold.
- Symptoms of eye irritation (burning, stinging, itching, tearing, redness), eyelid inflammation, corneal inflammation, blurred vision, and retinal detachment, which can cause vision problems, decreased corneal sensitivity, dry eyes, corneal ulcers (damage to the outer surface of the eyeball), drooping of the upper eyelid (causing the eyes to be half-closed), double vision.
- Darkening of the skin around the eyes, changes in eyelashes and fine hairs around the eye (increased number, length, thickness, and darkening), changes in the direction of eyelash growth, swelling around the eyes, swelling of the colored part of the eye (uveitis/iritis), scarring of the eye surface.
- Ringing or buzzing in the ears (tinnitus).
- Chest pain, worsening of chest pain in patients with existing heart disease.
- Slow heart rate, palpitations (awareness of heart rhythm), swelling (fluid accumulation), arrhythmias, heart failure, irregular heart rhythm, cardiac arrest, heart failure.
- Low blood pressure, poor blood circulation causing fingers and toes to become numb and pale, and hands and feet to become cold.
- Shallow breathing, narrowing of the airways in the lungs (usually in patients with pre-existing disease), breathing difficulties, cough, asthma, worsening of asthma.
- Taste disturbances, nausea, indigestion, diarrhea, dry mouth, stomach pain, vomiting.
- Hair loss, rash with white-silvery patches (psoriasis-like) or worsening of psoriasis symptoms, skin rash.
- Joint pain, muscle pain not caused by physical exertion, muscle weakness, fatigue.
- Sexual dysfunction, decreased libido.
Very rarely, in some patients with significantly damaged transparent front part of the eye (cornea), cloudy spots on the cornea have developed due to calcium deposition during treatment.
Reporting side effects
If the patient experiences any side effects, including those not listed in the leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Akistan DUO
The medicine should be stored out of sight and reach of children.
Akistan DUO should not be used after the expiry date stated on the outer packaging and bottle after EXP. The expiry date refers to the last day of the month.
Unopened bottles of Akistan DUO should be stored in the refrigerator (between 2°C and 8°C). Do not freeze.
After opening the bottle, it is not necessary to store the medicine in the refrigerator, but it should not be stored above 25°C. After the first opening of the bottle, the medicine is suitable for use for 4 weeks.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Akistan DUO contains
The active substances of Akistan DUO are latanoprost at a concentration of 50 micrograms/ml and timolol (as maleate) at a concentration of 5 mg/ml.
The other ingredients are sodium chloride, benzalkonium chloride 10% (w/w), sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, phosphoric acid 10% (w/v), and/or sodium hydroxide 10% (w/v) (to adjust the pH to 5.5-6.5), purified water.
What Akistan DUO looks like and what the pack contains
Akistan DUO is a sterile, clear, colorless liquid.
Akistan DUO is available in packs of 1, 3, and 6 bottles. One bottle contains 2.5 ml of Akistan DUO solution. The approximate number of drops in a bottle is 80 drops.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Pharmaselect International Beteiligungs GmbH
Ernst-Melchior-Gasse 20
1020 Vienna
Austria
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Bulgaria, Czech Republic, Poland, Slovakia, Hungary:
Akistan DUO
Belgium, Slovenia:
AKISTANCOMB
Netherlands: BEKISTAN
Date of last revision of the leaflet:March 2022
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterPharmaselect International Beteiligungs GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Akistan DuoDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsPrescription required
Alternatives to Akistan Duo in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Akistan Duo in Spain
Alternative to Akistan Duo in Ukraine
Online doctors for Akistan Duo
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Akistan Duo – subject to medical assessment and local rules.














