
Vizilatan Duo

Ask a doctor about a prescription for Vizilatan Duo

How to use Vizilatan Duo
Leaflet accompanying the packaging: patient information
Vizilatan Duo, 50 micrograms/ml 5 mg/ml,
eye drops, solution
Latanoprost + Timolol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor or pharmacist. This also applies to any side effects not listed in this leaflet. See section 4.
Table of contents of the leaflet
- 1. What is Vizilatan Duo and what is it used for
- 2. Important information before using Vizilatan Duo
- 3. How to use Vizilatan Duo
- 4. Possible side effects
- 5. How to store Vizilatan Duo
- 6. Contents of the packaging and other information
1. What is Vizilatan Duo and what is it used for
Vizilatan Duo contains two active substances: latanoprost and timolol. Latanoprost belongs to a group
of medicines called prostaglandin analogues. Timolol belongs to a group of medicines called beta-
adrenergic blockers. Latanoprost works by increasing the outflow of fluid from the eye into the
bloodstream. Timolol works by reducing the amount of fluid produced in the eye.
Vizilatan Duo is used to reduce intraocular pressure in patients with open-angle glaucoma or
increased intraocular pressure. Both conditions are associated with increased intraocular pressure and
can affect vision. The doctor will usually recommend using Vizilatan Duo if the action of other medicines
has not been sufficient.
Vizilatan Duo can be used in adults, including the elderly, but it should not be used in children and
adolescents under 18 years of age.
Vizilatan Duo, eye drops, is a sterile solution that does not contain preservatives.
2. Important information before using Vizilatan Duo
When not to use Vizilatan Duo
- if the patient is allergic (hypersensitive) to the active substances (latanoprost or timolol), beta-adrenergic blockers, or any of the other ingredients of this medicine (listed in section 6).
- if the patient has or has had respiratory problems, such as asthma, severe obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, and/or prolonged coughing).
- if the patient has severe heart problems or rhythm disorders.
Warnings and precautions
Before starting to use Vizilatan Duo, the patient should inform their doctor or pharmacist if they have or
have had:
- coronary heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, low blood pressure
- heart rhythm disorders, such as slow heart rate
- breathing difficulties, asthma, or chronic obstructive pulmonary disease
- diseases related to poor blood circulation (such as Raynaud's disease or Raynaud's syndrome)
- diabetes, as timolol may mask the symptoms of low blood sugar
- hyperthyroidism, as timolol may mask the symptoms
- if the patient is scheduled to have eye surgery (including cataract surgery) or if any eye surgery has been performed in the past
- if the patient has eye problems (such as eye pain, irritation of the eye surface, conjunctivitis, or blurred vision)
- if the patient has dry eyes
- if the patient uses contact lenses. Vizilatan Duo can still be used, but the patient should follow the instructions for using contact lenses contained in section 3
- if the patient has angina (especially a type called Prinzmetal's angina)
- if the patient has severe allergic reactions that usually require hospital treatment
- if the patient has had or currently has a viral eye infection caused by the Herpes Simplexvirus (HSV).
Before surgery, the patient should inform their doctor about using Vizilatan Duo, as timolol may affect the action of certain medicines used during anesthesia.
Children and adolescents
Vizilatan Duo should not be used in patients under 18 years of age.
Vizilatan Duo and other medicines
The patient should tell their doctor or pharmacist about all medicines (including eye drops) they are
currently taking or have recently taken, as well as any medicines they plan to take; including those
available without a prescription.
Vizilatan Duo may interact with and be affected by other medicines, including eye drops used to
treat glaucoma. The patient should inform their doctor about using or planning to use medicines that
lower blood pressure, heart medicines, or medicines used to treat diabetes.
In particular, the patient should inform their doctor or pharmacist if they are using a medicine that
belongs to any of the following groups:
- Prostaglandins, prostaglandin analogues, or prostaglandin derivatives (used to stimulate and inhibit smooth muscle contractions, dilate and constrict blood vessels, control blood pressure, and modulate the state of inflammation)
- Beta-adrenergic blockers (used to treat high blood pressure, angina, certain heart rhythm disorders, heart attack, anxiety, migraine, glaucoma, and hyperthyroidism)
- Epinephrine (used to treat life-threatening allergic reactions caused by insect bites or stings, foods, medicines, latex, and other factors)
- Blood pressure-lowering medicines such as oral calcium channel blockers, guanethidine, antiarrhythmic medicines, cardiac glycosides, or parasympathomimetics
- Quinidine (used to treat heart disorders and certain types of malaria)
- Antidepressants, such as fluoxetine, paroxetine.
Vizilatan Duo with food and drink
Normal meals, food, or drinks do not affect when and how to use Vizilatan Duo.
Pregnancy, breastfeeding, and fertility
Pregnancy
Vizilatan Duo should not be used during pregnancy, unless the doctor decides it is necessary. If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
Breastfeeding
Vizilatan Duo should not be used during breastfeeding. Vizilatan Duo may pass into breast milk. Before using any medicine during breastfeeding, the patient should consult their doctor.
Fertility
In animal studies, no effect of latanoprost and timolol on male or female fertility has been found.
Driving and using machines
After using Vizilatan Duo, blurred vision may occur for a short period. If the patient experiences this type of discomfort, they should not drive or operate machines until the discomfort resolves.
Vizilatan Duo contains macrogolglycerol hydroxystearate 40
This medicine contains macrogolglycerol hydroxystearate 40, which may cause skin reactions.
Vizilatan Duo contains phosphate buffer
This medicine contains 0.18 mg of phosphates in each drop, which corresponds to 6.43 mg/ml.
In patients with severe damage to the transparent, front part of the eye (cornea), phosphates may
very rarely cause corneal clouding due to calcium accumulation during treatment.
3. How to use Vizilatan Duo
This medicine should always be used exactly as prescribed by the doctor or pharmacist. If the patient
has any doubts, they should consult their doctor or pharmacist.
The recommended dose for adults (including the elderly) is 1 drop into the affected eye(s) once a day.
Vizilatan Duo should not be used more frequently than once a day, as this may reduce the effectiveness
of the treatment.
Vizilatan Duo should be used as directed by the doctor, who will decide when to stop the treatment.
If the patient is using Vizilatan Duo, the doctor may recommend additional heart and circulatory system
examinations.
Using contact lenses
If the patient uses contact lenses, they should remove them before using Vizilatan Duo. After using
Vizilatan Duo, the patient should wait 15 minutes before reinserting the contact lenses.
Instructions for using Vizilatan Duo
1a 1b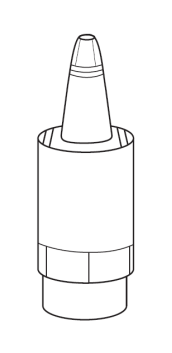 |
|
2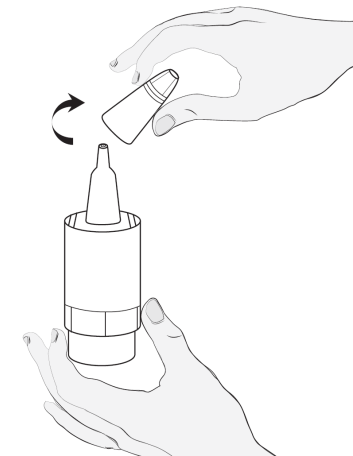 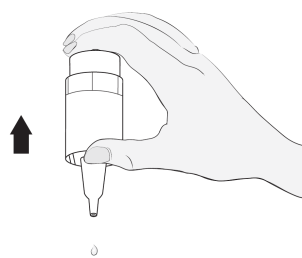 |
|
3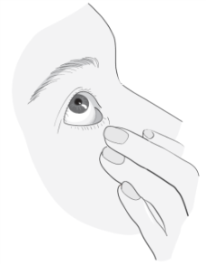 |
|
4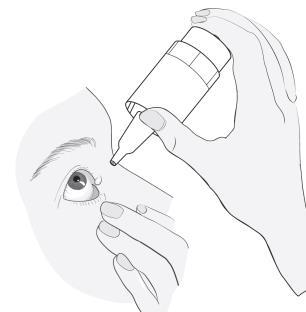 |
|
5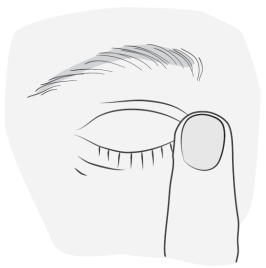 |
|
|
Using Vizilatan Duo with other eye drops
After administering Vizilatan Duo, wait at least 5 minutes before administering other eye drops.
Using a higher dose of Vizilatan Duo than recommended
In case of administering a larger amount of eye drops, moderate eye irritation may occur, with tearing and redness. These symptoms should resolve, but if the patient is concerned, they should consult their doctor.
Accidental ingestion of Vizilatan Duo
In case of accidental ingestion of Vizilatan Duo, the patient should consult their doctor. In case of ingestion of a large amount of eye drops, Vizilatan Duo may cause malaise, abdominal pain, fatigue, redness of the face, and dizziness, as well as sweating.
Missing a dose of Vizilatan Duo
If a dose is missed, the patient should take the next dose at the scheduled time. The patient should not take a double dose to make up for the missed dose. If the patient has any doubts, they should consult their doctor or pharmacist.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Vizilatan Duo can cause side effects, although not everybody gets them.
Usually, the patient can continue using the eye drops, unless the side effects are severe. If the patient has any doubts, they should consult their doctor or pharmacist. The patient should not stop using Vizilatan Duo without consulting their doctor.
The following are known side effects that have occurred after using a medicine containing latanoprost and timolol as active substances. The most important side effect is the possibility of gradual, permanent changes in eye color. Vizilatan Duo eye drops (containing latanoprost and timolol) may cause serious changes in heart function. The patient should inform their doctor if they notice changes related to heart rate or heart function and inform them about using Vizilatan Duo.
Known side effects that have occurred after using eye drops containing latanoprost and timolol as active substances:
Very common(may affect more than 1 in 10 people):
- Gradual change in eye color associated with an increase in the amount of brown pigment in the colored part of the eye called the iris. In patients with mixed eye color (such as blue-brown, gray-brown, yellow-brown, or green-brown), these changes can be noticed much more often than in people with uniform eye color (blue, gray, green, or brown). Eye color changes may develop over several years. The change in eye color may be permanent and more noticeable if only one eye is treated with Vizilatan Duo. The change in eye color appears to be harmless. After stopping the use of Vizilatan Duo, the change in eye color does not progress.
Common(may affect up to 1 in 10 people):
- Eye irritation (including a feeling of burning, grittiness, itching, stinging, or a feeling of a foreign body in the eye), eye pain.
Uncommon(may affect up to 1 in 100 people):
- Headache,
- Redness of the eye, eye infection (conjunctivitis), blurred vision, increased tearing, eyelid inflammation, irritation or damage to the eye surface
- Skin rash, itching (pruritus).
Other side effects
Like other eye medicines, Vizilatan Duo (latanoprost with timolol) is absorbed into the bloodstream. The frequency of side effects after using eye drops is lower than with medicines taken orally or intravenously.
The following are side effects that, although they did not occur after using a medicine containing both latanoprost and timolol, did occur after using individual components of Vizilatan Duo, and therefore may also occur after using Vizilatan Duo.
Among the listed side effects are also side effects observed during the use of beta-adrenergic blockers (e.g., timolol) in the treatment of eye diseases.
- Development of eye inflammation caused by herpes simplex virus (HSV) infection
- General allergic reactions, including swelling under the skin, which can occur on the face, limbs, and can make breathing difficult, causing difficulty swallowing and breathing, hives, or itchy rash, local and generalized rash, itching, severe life-threatening allergic reactions
- Low blood sugar
- Dizziness
- Difficulty sleeping (insomnia), depression, nightmares, memory loss, hallucinations
- Fainting, stroke, decreased blood flow to the brain, worsening of myasthenia symptoms (muscle weakness), feeling of tingling or numbness and headache
- Posterior eye swelling (macular edema), formation of fluid-filled spaces in the colored part of the eye (iris cysts), sensitivity to light (photophobia), feeling of sunken eyes (deepening of the eyelid fold)
- Symptoms of eye irritation (burning, stinging, itching, tearing, redness), eyelid inflammation, conjunctivitis, corneal inflammation, blurred vision, and detachment of the retina, which can be a complication of surgical filtration and can cause vision disturbances, decreased corneal sensitivity, dry eye, corneal ulcers (loss of the outer surface of the eyeball), drooping of the upper eyelid (causing the eyes to be half-closed), double vision
- Darkening of the skin around the eyes, changes in eyelashes and primary hair around the eyes (increased
number, length, thickness, and darkening), misdirected eyelashes, swelling around the eyes,
swelling of the iris - the colored part of the eye (iritis and/or uveitis), scarring conjunctivitis
U pacjentów z ciężkimi uszkodzeniami przezroczystej, przedniej części oka (rogówki), fosforany mogą
w bardzo rzadkich przypadkach spowodować w czasie leczenia zmętnienia rogówki z powodu
gromadzenia się wapnia.
Reporting side effects
If side effects occur, including those not listed in the leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: + 48 22 49 21 301 fax: + 48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Vizilatan Duo
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month stated.
There are no special precautions for storing this medicine.
The medicine should be discarded after 4 weeks from the first opening of the bottle,to prevent infection.
The patient should write the opening date of the bottle in the designated space on the carton.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Vizilatan Duo contains
- The active substances of Vizilatan Duo are latanoprost and timolol. Each ml of eye drops contains 50 micrograms of latanoprost and 5 mg of timolol (in the form of 6.8 mg of timolol maleate).
- The other ingredients are: macrogolglycerol hydroxystearate 40, sodium chloride, disodium edetate, sodium dihydrogen phosphate dihydrate, disodium phosphate, hydrochloric acid, and/or sodium hydroxide (to adjust the pH), water for injections.
What Vizilatan Duo looks like and what the pack contains
Vizilatan Duo is a clear, colorless aqueous solution with a volume of 2.5 ml, in a white multidose container (HDPE) with a capacity of 5 ml, with a pump (PP, HDPE, LDPE) and an orange pressure cylinder and cap (HDPE). The container is placed in a cardboard box.
Pack size: 1, 3, or 4 bottles of 2.5 ml.
Not all pack sizes may be marketed.
Marketing authorization holder:
Bausch+Lomb Ireland Limited
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24 PPT3
Ireland
Manufacturer:
Pharmathen S.A.
Dervenakion 6
15351 Pallini Attiki
Greece
Lomapharm GmbH
Langes Feld 5
31860 Emmerthal
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Vizilatan Duo 0.05 mg/ml + 5 mg/ml eye drops, solution
Belgium
Vizilaticom 50 micrograms/ml + 5 mg/ml eye drops, solution / Vizilaticom 50 micrograms/ml + 5 mg/ml, eye drops in solution / Vizilaticom 50 micrograms/ml + 5 mg/ml eye drops, solution
Denmark
Vizilatan Duo
Estonia
Vizilatan Duo
Luxembourg
Vizilaticom
Germany
Vizilatan Duo 0.05 mg/ml + 5 mg/ml eye drops, solution
France
KILATIM 50 micrograms/ml + 5 mg/ml, eye drops in solution
Spain
Vizilatan Duo 0.05 mg/ml + 5 mg/ml eye drops, solution
Netherlands
Vizilaticom 50 micrograms/ml + 5 mg/ml eye drops, solution
Poland
Vizilatan Duo
Portugal
Vizilatan Duo 0.05 mg/ml + 5 mg/ml eye drops, solution
Czech Republic
Vizilatan Duo
Romania
Vizilatan Duo 0.05 mg/ml + 5 mg/ml eye drops, solution
Hungary
Vizilatan Duo 0.05 mg/ml + 5 mg/ml eye drops, solution
Italy
Vizilatan
Date of last revision of the leaflet: 06.2022
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterLomapharm GmbH Pharmathen S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Vizilatan DuoDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Vizilatan Duo in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Vizilatan Duo in Spain
Alternative to Vizilatan Duo in Ukraine
Online doctors for Vizilatan Duo
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Vizilatan Duo – subject to medical assessment and local rules.














