
Tobradex

Ask a doctor about a prescription for Tobradex

How to use Tobradex
Leaflet attached to the packaging: patient information
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Tobradex
(3 mg + 1 mg)/ml, eye drops, suspension
Tobramycin + Dexamethasone
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for one person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Tobradex and what is it used for
- 2. Important information before using Tobradex
- 3. How to use Tobradex
- 4. Possible side effects
- 5. How to store Tobradex
- 6. Contents of the packaging and other information
1. What is Tobradex and what is it used for
Tobradex is used to treat inflammatory eye diseases, which may be accompanied by
infection.Eye inflammation can result from eye surgery, infection, or be caused by the presence of a foreign body or eye injury.
Tobradex is a combination drug containing an antibacterial component and
corticosteroid. Corticosteroids (in this case, dexamethasone) are used to prevent and reduce eye inflammation. The antibacterial drug contained in the medicine (tobramycin) acts on many species of pathogenic bacteria that infect the eye.
The indication for using Tobradex is to prevent and treat inflammation and prevent infections related to cataract surgery in adults and children over 2 years of age.
2. Important information before using Tobradex
When not to use Tobradex:
- if the patient is allergic to dexamethasone, tobramycin, or any of the other ingredients of this medicine (listed in section 6); or if the patient has:
- herpes simplex virus keratitis;
- keratitis caused by the cowpox virus, chickenpox/herpes zoster, and other viral diseases of the cornea or conjunctiva;
- eye tuberculosis;
- fungal eye infection or untreated parasitic eye infections;
- untreated purulent eye infections;
- if the patient has had a foreign body removed from the cornea and no complications are present.
Warnings and precautions
- If the patient experiences allergic reactions after using Tobradex, they should discontinue treatment and consult a doctor immediately (see section 4). Hypersensitivity symptoms can have varying severity: from local itching or skin redness to severe allergic reactions (anaphylactic reaction) or severe skin reactions. Such allergic reactions may occur when using other topical or systemic antibiotics from the same group (aminoglycosides).
- If the patient's symptoms worsen or suddenly recur, they should consult a doctor. During the use of this medicine, the patient may be more susceptible to eye infections.
- If the patient is using other antibiotics, including those taken orally, they should consult a doctor.
- If the patient has or suspects myasthenia or Parkinson's disease, they should consult a doctor. Antibiotics from this group may exacerbate muscle weakness.
- If the patient uses Tobradex for a longer period, they may experience:
- increased susceptibility to eye infections,
- increased intraocular pressure,
- development of cataracts,
- development of Cushing's syndrome due to the absorption of the medicine into the bloodstream. The patient should consult a doctor if they experience swelling and weight gain, particularly in the trunk and face, as these are usually the first symptoms of Cushing's syndrome. Adrenal insufficiency may occur after discontinuation of long-term or intensive use of Tobradex. The patient should consult a doctor before deciding to discontinue treatment. This risk is particularly important in children and patients treated with ritonavir or cobicistat.
- During the use of Tobradex, the patient should regularly check their intraocular pressure; they should consult a doctor. This is especially important in children, as the risk of developing ocular hypertension caused by corticosteroids may be higher in children and may occur faster than in adults. Especially in the case of children and adolescents, the patient should consult a doctor. The risk of ocular hypertension and cataract formation is also higher in patients with other diseases (e.g., patients with diabetes).
- Steroid eye drops can cause delayed healing of eye injuries. Topically applied non-steroidal anti-inflammatory drugs (NSAIDs) can slow down and delay the healing process. Concurrent topical use of non-steroidal anti-inflammatory drugs and steroid drugs can cause a risk of eye healing problems.
- If the patient has a disease that causes thinning of the eye tissues, they should consult a doctor or pharmacist.
- If the patient experiences persistent corneal ulcers during the use of Tobradex, they should consult a doctor as soon as possible, as this may be a sign of fungal eye infection.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Tobradex and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
The patient should especially tell their doctor if they are using non-steroidal anti-inflammatory drugs (NSAIDs).
Concurrent topical use of a steroid medicine and a non-steroidal anti-inflammatory drug may cause problems with eye healing.
If the patient is using other eye drops or ointments, they should leave a 5-minute interval between administrations of the different medicines. Eye ointments should be used last.
The patient should tell their doctor about taking ritonavir or cobicistat, as these medicines may increase the dexamethasone content in the blood.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor before using this medicine.
Tobradex is not recommended during pregnancy and breastfeeding.
Driving and using machines
Tobradex has no or negligible influence on the ability to drive and use machines.
For some time after administering Tobradex, vision may be blurred. The patient should not drive or operate machines until this symptom subsides.
Tobradex contains benzalkonium chloride
The medicine contains 0.1 mg of benzalkonium chloride per milliliter (0.1 mg/ml).
Benzalkonium chloride may be absorbed by soft contact lenses and change their color. The patient should remove contact lenses before administering the drops and wait at least 15 minutes before putting them back.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer at the front of the eye). If the patient experiences abnormal sensations in the eye, stinging, or eye pain after using the medicine, they should contact their doctor.
3. How to use Tobradex
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult a doctor or pharmacist.
The dosage of Tobradex is determined individually by the doctor for each patient. The doctor will decide how long the medicine should be used. If the doctor does not recommend otherwise, the patient should instill one to two drops of the medicine into the conjunctival sac (sacs) of the infected eye (eyes) every 4-6 hours.
Use in children
The medicine can be used in children over 2 years of age in the same doses as in adults.
The safety and efficacy in children under 2 years of age have not been established, and there are no data available for this age group.
Use in patients with liver or kidney impairment
The effect of Tobradex has not been studied in these patient populations. However, due to the low systemic absorption of tobramycin and dexamethasone after local administration, it is considered that there is no need to modify the dosage.
Tobradex is intended exclusivelyfor eye drops.
1
2
3
4
- 1. Prepare the Tobradex bottle and a mirror.
- 2. Wash your hands.
- 3. Shake the bottle.
- 4. Remove the cap. If the protective collar is loose after removing the cap, it should be discarded before using the medicine.
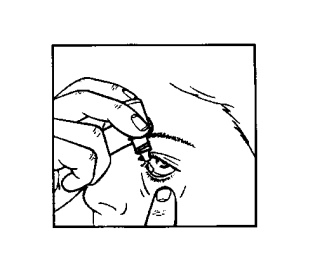
- 5. Hold the bottle in your hand and turn it upside down, holding it with your thumb and middle finger (drawing 1).
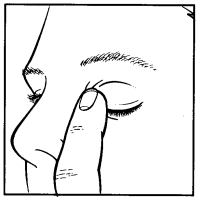
- 6. Tilt your head back. Pull down the lower eyelid with a clean finger to create a "pocket" between the eyelid and the eyeball; the drop should fall into this pocket (drawing 2).
- 7. Bring the dropper tip close to the eye. You can use a mirror to help.
- 8.
Do not touch the dropper tip to the eye, eyelid, or surrounding areas.
Failing to follow this recommendation may cause infection of the drops. Using infected drops can lead to dangerous complications, even vision loss.
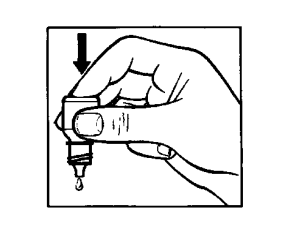
- 9. Gently squeeze the bottom of the bottle to release a single drop of Tobradex (drawing 3). If the drop does not fall into the eye,you should repeat the attempt.
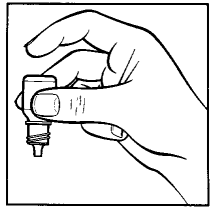
- 10. After administering Tobradex, release the lower eyelid. Gently close your eye and press the corner of your eye near your nose with your finger (drawing 4). This helps prevent the medicine from entering the entire body.
- 11. If it is necessary to administer the medicine to both eyes, you should repeat the above steps for the second eye.
- 12. Immediately after using the medicine, tighten the bottle cap.
- 13. You should use only one bottle of the medicine at a time.
Using more than the recommended dose of Tobradex
In case of overdose, excess medicine can be flushed from the eye with lukewarm water. Do not administer additional drops. The next dose should be administered at the usual time.
Missing a dose of Tobradex
If the patient forgets to use Tobradex, they should continue treatment by administering the next dose according to the dosage schedule. If it is almost time for the next dose, the patient should skip the missed dose and continue treatment according to the recommended dosage schedule. The patient should not use a double doseto make up for the missed dose.
In case of any further doubts about using this medicine, the patient should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Tobradex can cause side effects, although not everybody gets them.
If the patient experiences allergic reactions, including rash, face, lip, tongue, and (or)
throat swelling, which may cause difficulty breathing or swallowing, or other severe
side effects, they should discontinue using Tobradex and contact their doctor or the Emergency Department of the nearest hospital immediately.
During the use of Tobradex, the following side effects have been observed.
Uncommon( may occur in less than 1 in 100 patients): high intraocular pressure, eye pain, eye itching, eye discomfort, eye irritation.
Rare( may occur in less than 1 in 1,000 patients): keratitis, eye allergy, blurred vision, dry eye syndrome, redness, taste disorders.
Frequency not known( frequency cannot be estimated from the available data): eyelid swelling, eyelid redness, pupil dilation, increased tearing, blurred vision, severe allergic reactions (hypersensitivity), dizziness, headache, nausea, abdominal discomfort, severe skin reactions (erythema multiforme), itching, face swelling, increased hair growth on the body (especially in women), muscle weakness, and muscle mass loss, purple striae on the skin, increased blood pressure, irregular menstrual periods or amenorrhea, changes in protein and calcium levels in the body, growth retardation in children and adolescents, as well as swelling and weight gain, particularly in the trunk and face (a condition known as Cushing's syndrome) (see section 2 "Warnings and precautions").
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Tobradex
To avoid infection of the medicine, the bottle should be discarded 4 weeks after first opening. The patient should write the date of opening the bottle in the space provided below.
Date of first opening: ………….
The medicine should be stored out of sight and reach of children.
Store at a temperature below 25°C. Do not freeze.
Store the bottle upright. Store the bottle tightly closed.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the stated month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Tobradex contains
- The active substances of the medicine are tobramycin 3 mg/ml and dexamethasone 1 mg/ml.
- The other ingredients are benzalkonium chloride, disodium edetate, sodium chloride, anhydrous sodium sulfate (E 514), tyloxapol, hydroxyethylcellulose, purified water. The medicine may contain minimal amounts of sulfuric acid and/or sodium hydroxide (to adjust the pH)
What Tobradex looks like and what the packaging contains
Tobradex is a liquid (suspension of white to off-white color) available in 5 ml bottles (LDPE) with a dropper (LDPE) and a cap (PP), in a cardboard box.
For more detailed information, the patient should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Bulgaria, the country of export:
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Manufacturer:
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Alcon-Couvreur NV, Rijksweg 14, 2870 Puurs, Belgium
Siegfried El Masnou, S.A., Camil Fabra, 58, El Masnou, 08320, Barcelona, Spain
Novartis Pharma GmbH, Roonstrasse 25, 90429 Nuremberg, Germany
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Marketing authorization number in Bulgaria, the country of export:9600064
Parallel import authorization number:31/15
Date of leaflet approval: 20.12.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Novartis Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TobradexDosage form: Drops, (3 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Rafarm S.A.Prescription requiredDosage form: Drops, (5 mg + 1 mg)/mlActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription requiredDosage form: Ointment, (5 mg + 0.3 mg)/gActive substance: dexamethasone and antiinfectivesManufacturer: Dr. Gerhard Mann Chem. - Pharm. Fabrik GmbHPrescription required
Alternatives to Tobradex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tobradex in Украина
Alternative to Tobradex in Испания
Online doctors for Tobradex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tobradex – subject to medical assessment and local rules.














