
Tiotropium Adoh
Ask a doctor about a prescription for Tiotropium Adoh

How to use Tiotropium Adoh
PATIENT INFORMATION LEAFLET
Patient Information Leaflet: Information for the User
Tiotropium ADOH, 18 micrograms, inhalation powder in a hard capsule
Tiotropium
Read the contents of the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Contents of the Leaflet
- 1. What is Tiotropium ADOH and what is it used for
- 2. Important information before using Tiotropium ADOH
- 3. How to use Tiotropium ADOH
- 4. Possible side effects
- 5. How to store Tiotropium ADOH
- 6. Contents of the pack and other information
1. What is Tiotropium ADOH and what is it used for
Tiotropium ADOH helps people with chronic obstructive pulmonary disease (COPD) to breathe more easily. COPD is a chronic lung disease that causes shortness of breath and coughing. COPD is associated with chronic bronchitis and emphysema. Because COPD is a chronic disease, Tiotropium ADOH should be taken every day, and not just when breathing difficulties or other COPD symptoms occur. Tiotropium ADOH is a long-acting bronchodilator that helps to open up the airways and make it easier for air to get in and out of the lungs. Regular use of this medicine can also help to reduce the severity of symptoms associated with the disease. This medicine will help minimize the impact of the disease on the patient's daily life. The medicine also helps the patient to be more active. Daily use of Tiotropium ADOH will also help prevent sudden, short-term worsening of COPD symptoms that can last for several days. The effect of this medicine lasts for 24 hours, so it should be taken only once a day. The correct dosage of the medicine is described in section 3, "How to use Tiotropium ADOH," and the instructions for use are provided at the end of this leaflet.
2. Important information before using Tiotropium ADOH
When not to use Tiotropium ADOH
- if the patient is allergic (hypersensitive) to tiotropium, the active substance, or any of the other ingredients of this medicine (listed in section 6).
- if the patient is allergic (hypersensitive) to atropine or its derivatives, such as ipratropium or oxytropium.
Warnings and precautions
Before starting treatment with Tiotropium ADOH, the patient should discuss it with their doctor or pharmacist.
The patient should consult their doctor if they:
- have narrow-angle glaucoma (an eye disease),
- have problems with the prostate gland,
- have difficulty urinating,
- have kidney problems. This medicine is indicated for the maintenance treatment of COPD and should not be used to treat sudden attacks of shortness of breath or wheezing.
The patient should immediately consult their doctor if they experience immediate allergic reactions, such as rash, swelling, itching, wheezing, or shortness of breath. This can occur after administration of this medicine. The patient should immediately consult their doctor if they experience chest tightness, coughing, wheezing, or shortness of breath shortly after inhaling this medicine. The patient should be careful not to get the inhalation powder in their eyes, as it may cause eye irritation or worsening of narrow-angle glaucoma (an eye disease). The patient should stop using Tiotropium ADOH and immediately consult their doctor, preferably an eye specialist, if they experience signs and symptoms of narrow-angle glaucoma. Eye pain, blurred vision, seeing halos (auras) around lights, or colored images associated with eye redness may be a sign of acute narrow-angle glaucoma. Eye symptoms may be accompanied by headache, nausea, or vomiting. The patient should pay attention to oral hygiene. Dry mouth, which has been observed with anticholinergic treatment, may be associated with tooth decay in the long term. The patient should inform their doctor if they have had a heart attack in the last 6 months or unstable or life-threatening irregular heartbeat or severe heart failure in the last year. It is essential to decide whether this medicine is suitable for the patient to take. The patient should not use Tiotropium ADOH more often than once a day.
Children and adolescents
Tiotropium ADOH is not recommended for children and adolescents under 18 years of age.
Tiotropium ADOH and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, including those obtained without a prescription. The patient should tell their doctor or pharmacist if they are using similar medicines for their lung disease, such as ipratropium or oxytropium. No specific interactions have been reported when this medicine is used with other medicines for the treatment of COPD, such as relief inhalers, such as salbutamol, methylxanthines, such as theophylline, and/or oral steroids, such as prednisolone.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should ask their doctor or pharmacist for advice before using this medicine. The patient should not use this medicine unless their doctor advises them to.
Driving and using machines
Dizziness, blurred vision, or headache may affect the patient's ability to drive or operate machinery.
Tiotropium ADOH contains lactose
When used as directed, one capsule provides up to 12.48 mg of lactose monohydrate. If the doctor has informed the patient that they have an intolerance to some sugars or an allergy to milk proteins (which may be present in small amounts in the lactose monohydrate ingredient), the patient should contact their doctor before taking the medicine.
3. How to use Tiotropium ADOH
This medicine should always be used as directed by the doctor or pharmacist. If the patient is unsure, they should ask their doctor or pharmacist. The recommended dose is the inhalation of the contents of one capsule (18 micrograms of tiotropium) once a day. The patient should not use more than the recommended dose. Tiotropium ADOH is not recommended for children and adolescents under 18 years of age. The medicine should be used every day at the same time. This is important because it works for 24 hours. The capsules are for inhalation only and should not be swallowed. The RS01 inhaler, into which the Tiotropium ADOH capsule should be inserted, makes holes in the capsule and allows the powder to be inhaled. The patient should ensure that the RS01 inhaler is available and can be used properly. The instructions for cleaning the RS01 inhaler are provided at the end of this leaflet. The patient should ensure that they do not blow into the RS01 inhaler. If the patient has problems using the RS01 inhaler, they should ask their doctor, nurse, or pharmacist to show them how it works. When taking the medicine, the patient should be careful not to get the powder in their eyes. If any powder gets into the eyes, it may cause blurred vision, pain, and/or eye redness. The patient should immediately rinse their eyes with warm water. Then, the patient should consult their doctor as soon as possible for further advice. If the patient experiences worsening of breathing, they should consult their doctor as soon as possible.
Using a higher dose of Tiotropium ADOH than recommended
If the patient inhales more than one Tiotropium ADOH capsule in a day, they should consult their doctor immediately. The patient may be at a higher risk of experiencing side effects, such as dry mouth, constipation, difficulty urinating, rapid heartbeat, or blurred vision.
Missing a dose of Tiotropium ADOH
If the patient forgets to take a dose, they should take it as soon as they remember, but not take two doses at the same time or on the same day. Then, the patient should take the next dose as usual.
Stopping treatment with Tiotropium ADOH
Before stopping treatment with this medicine, the patient should consult their doctor or pharmacist. If the patient stops using the medicine, their COPD symptoms may worsen. If the patient has any further questions about using this medicine, they should ask their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects listed below have been reported by people taking this medicine and are listed according to their frequency as common, uncommon, rare, or unknown.
Severe side effects
Severe side effects include allergic reactions that cause swelling of the face or throat (angioedema) or other hypersensitivity reactions (such as a sudden drop in blood pressure or dizziness), which can occur as part of a severe allergic reaction (anaphylaxis) after administration of Tiotropium ADOH, 18 micrograms. As with all inhaled medicines, some patients may experience unexpected chest tightness, coughing, wheezing, or shortness of breath shortly after inhalation (bronchospasm). If any of these events occur, the patient should consult their doctor. The side effects listed below have been reported by people taking this medicine and are listed according to their frequency as common, uncommon, rare, or unknown.
Common (may affect up to 1 in 10 people):
- dry mouth, which is usually mild
Uncommon (may affect up to 1 in 100 people):
- dizziness
- headache
- taste disturbances
- blurred vision
- irregular heartbeat (atrial fibrillation)
- throat irritation
- hoarseness
- cough
- heartburn (gastroesophageal reflux disease)
- constipation
- fungal infections of the mouth and throat (oropharyngeal candidiasis)
- rash
- difficulty urinating (urinary retention)
- painful or difficult urination
Rare (may affect up to 1 in 1000 people):
- difficulty sleeping (insomnia)
- seeing halos (auras) around lights or colored images associated with eye redness (glaucoma)
- increased measured pressure in the eye
- irregular heartbeat (supraventricular tachycardia)
- rapid heartbeat (tachycardia)
- feeling of heartbeat (palpitations)
- chest tightness associated with coughing, wheezing, or shortness of breath shortly after inhalation (bronchospasm)
- nasal bleeding
- throat irritation
- sinusitis
- blockage of the intestines or lack of intestinal movement (intestinal obstruction, including paralytic ileus)
- gum inflammation
- tongue inflammation
- difficulty swallowing
- mouth inflammation
- nausea
- hypersensitivity, including immediate reactions
- severe allergic reaction, which causes swelling of the face or throat (angioedema)
- hives
- itching
- urinary tract infections
Unknown frequency (frequency cannot be estimated from the available data):
- dehydration
- tooth decay
- severe allergic reaction (anaphylaxis)
- skin infections or ulcers
- dry skin
- joint swelling
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, PL-02 222 Warszawa, Tel.: + 48 22 49 21 301, Fax: + 48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, the patient can help provide more information on the safety of this medicine.
5. How to store Tiotropium ADOH
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiry date stated on the carton and bottle after EXP. The expiry date refers to the last day of that month. After removing the first capsule from the bottle, the patient should continue to use the capsules for the next 30 days. There are no special precautions for the storage of this medicinal product. Do not freeze. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Tiotropium ADOH contains
The active substance is tiotropium. Each capsule contains 18 micrograms of the active substance tiotropium (as monohydrate). During inhalation, 10 micrograms of tiotropium are delivered to the mouthpiece of the RS01 inhaler. The other ingredient is lactose monohydrate (which may contain small amounts of milk proteins).
What Tiotropium ADOH looks like and contents of the pack
Tiotropium ADOH 18 micrograms, inhalation powder in a hard capsule, is a transparent hard capsule.
The product is available in the following packs:
Pack containing 30 capsules in a bottle and an RS01 inhaler in a cardboard box.
Pack containing 60 capsules in two bottles and an RS01 inhaler in a cardboard box.
Pack containing 90 capsules in three bottles and an RS01 inhaler in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
ADOH B.V.
Godfried Bomansstraat 31
6543 JA Nijmegen
Netherlands
Tel. +31 24 379 29 36
Manufacturer:
Pharmadox Healthcare Ltd.
KW20A Kordin industrial Park
Paola PLA 3000
Malta
This medicine is authorized in the Member States of the European Economic Area
Germany
Tiotropium AAA-Pharma 18 Mikrogramm Hartkapseln mit Pulver zur Inhalation
Netherlands
Tiotropium ADOH 18 microgram, inhalatiepoeder in harde capsules
Poland
Tiotropium ADOH 18 mikrogramów, proszek do inhalacji w kapsułkach twardych
Date of last revision of the leaflet: 03/2025.
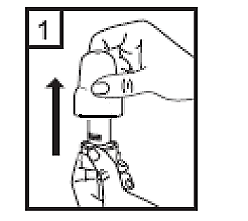
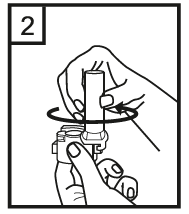
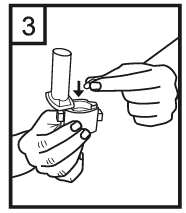
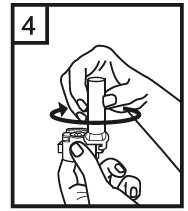
Instructions for use of the RS01 inhaler:
Dear patient,
The RS01 inhaler allows you to inhale the medicine contained in the Tiotropium ADOH capsule.
Please follow the instructions for use provided by your doctor. Do not take more than the recommended dose.
 |
|
 |
|
 |
|
 |
|
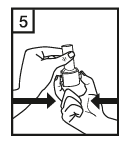 |
|
| |
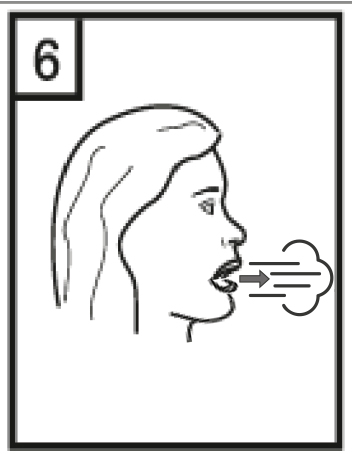 |
|
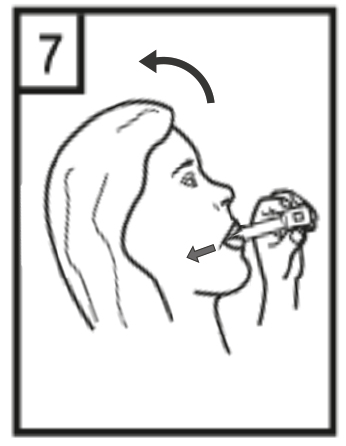 | |
| |
DO NOT use water to clean the inhaler. Then, close the mouthpiece and put the cap back on. After using the RS01 inhaler, the patient should always rinse their mouth and throat with water and spit out the remaining water. This can help prevent fungal infections in the mouth.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAdoh B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tiotropium AdohDosage form: Powder, 18 mcgActive substance: tiotropium bromideManufacturer: Ferrer Internacional, S.A.Prescription requiredDosage form: Powder, 10 mcgActive substance: tiotropium bromideManufacturer: Actavis Ltd. Laboratorios Liconsa S.A. Teva Operations Poland Sp. z o.o. Teva Pharma B.V.Prescription requiredDosage form: Powder, 18 mcg/measured doseActive substance: tiotropium bromidePrescription required
Alternatives to Tiotropium Adoh in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tiotropium Adoh in Ukraine
Alternative to Tiotropium Adoh in Spain
Online doctors for Tiotropium Adoh
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tiotropium Adoh – subject to medical assessment and local rules.














