
Braltus

Ask a doctor about a prescription for Braltus

How to use Braltus
Leaflet accompanying the packaging: information for the user
Braltus, 10 micrograms/delivered dose, powder for inhalation in a hard capsule
Tiotropium
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Braltus and what is it used for
- 2. Important information before using Braltus
- 3. How to use Braltus
- 4. Possible side effects
- 5. How to store Braltus
- 6. Contents of the packaging and other information
1. What is Braltus and what is it used for
Braltus contains the active substance tiotropium. Tiotropium makes it easier for people with chronic obstructive pulmonary disease (COPD) to breathe. COPD is a chronic lung disease that causes shortness of breath and coughing. The name COPD is associated with chronic bronchitis and emphysema. COPD is a chronic disease, so the medicine should be used every day, and not just when breathing problems or other COPD symptoms occur. Braltus is a long-acting bronchodilator that helps to widen the airways and make it easier for air to get in and out of the lungs. Regular use of this medicine can also help reduce persistent shortness of breath associated with the disease and help reduce the impact of the disease on daily life. The medicine helps to maintain activity for longer. Daily use of the medicine will also help prevent sudden, short-term worsening of COPD symptoms, which can last for several days. The effect of the medicine lasts for 24 hours, so it should only be used once a day. The medicine should not be used as a rescue treatment to relieve sudden chest tightness, coughing, or shortness of breath or wheezing. In such a situation, a fast-acting inhalation medicine for relief, such as salbutamol, should be used. You should always carry a fast-acting inhalation medicine for relief with you.
2. Important information before using Braltus
When not to use Braltus:
Warnings and precautions
Before starting to use Braltus, discuss with your doctor, pharmacist, or nurse if:
- you are taking other medicines containing ipratropium or oxytropium
- you have narrow-angle glaucoma, prostate problems, or difficulty urinating
- you have any kidney problems
- you have had a heart attack, experienced heart rhythm disturbances, or life-threatening irregular heartbeat, or severe heart failure in the last year. Braltus is indicated for maintenance treatment of chronic obstructive pulmonary disease. This medicine should not be used to treat sudden attacks of shortness of breath or wheezing.
During treatment with Braltus, immediate hypersensitivity reactions, such as rash, swelling, itching, wheezing, or shortness of breath, may occur. In such cases, you should immediatelycontact your doctor (see section 4). Inhalation medicines, such as Braltus, may cause chest tightness, coughing, wheezing, or shortness of breath (bronchospasm) immediately after use. In such situations, you should immediatelyuse a fast-acting relief inhalation medicine, such as salbutamol. If you experience any of these symptoms, you should stop using Braltus and immediatelycontact your doctor. You should not allow the inhalation powder to get into your eyes, as it may cause eye irritation or worsen glaucoma symptoms, which is an eye disease. Eye pain or discomfort, blurred vision, seeing a rainbow-colored ring around a light source, or changed color vision, along with eye redness, may be a sign of acute glaucoma. These eye problems may be accompanied by: headache, nausea, and vomiting. If you experience glaucoma symptoms, you should stop using this medicine and immediatelycontact your doctor, preferably an ophthalmologist. The medicine in the inhaler may reduce the amount of saliva (moisture) in the mouth and cause dry mouth. Over time, this may lead to tooth decay. Therefore, you should remember to maintain good oral hygiene, rinse your mouth, and brush your teeth regularly. If you have had a heart attack, experienced heart rhythm disturbances, or life-threatening irregular heartbeat, or severe heart failure in the last year, you should inform your doctor. This will allow your doctor to decide whether Braltus is a suitable treatment for you. You should notuse this medicine more often than once a day (see section 3).
Children and adolescents
Braltus should not be used in children and adolescents under 18 years of age.
Other medicines and Braltus
Tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, including inhalation medicines and medicines that are available without a prescription. You should inform your doctor or pharmacist about any other medicines you are currently taking or have taken in the past for lung disease, such as ipratropium or oxytropium. No specific interactions have been reported when using this medicine with other medicines used to treat COPD, such as: fast-acting inhalation medicines, e.g., salbutamol, methylxanthines (e.g., theophylline), and/or oral and inhalation steroids, e.g., prednisolone.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. You should not use this medicine unless your doctor tells you to.
Driving and using machines
Dizziness, blurred vision, or headache may affect your ability to drive or use machines.
Braltus contains lactose
Lactose is a type of sugar found in milk. If you have been diagnosed with an intolerance to some sugars, you should consult your doctor before taking this medicine. Lactose may contain small amounts of milk proteins, which may cause allergic reactions in people with severe hypersensitivity or milk protein allergy. If the medicine is used as directed, with a dose of one capsule once a day, each dose delivers up to 18 mg of lactose monohydrate.
3. How to use Braltus
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist. It is recommended to inhale the contents of onecapsule once a dayusing the Zonda inhaler. Onecapsule provides the required daily dose of tiotropium (delivered dose 10 micrograms tiotropium); do notuse more than the recommended dose. You should try to use the medicine at the same time every day. This is important because the medicine works for 24 hours. The capsules can only be used for inhalation. Do not swallow them. The Zonda inhaler, into which the Braltus capsule should be inserted, pierces the capsule and allows the powder to be inhaled. The capsules should only be administered using the Zonda inhaler. Do not use any other devices to administer Braltus capsules. You should make sure that you can use the Zonda inhaler correctly. The instructions for using the Zonda inhaler are on the other side of the leaflet. You should follow these instructions carefully. Pictures showing how to correctly place the capsule in the inhaler are also on the inside of the box flap. To avoid the risk of choking, NEVER insert the capsule directly into the mouthpiece.If you have any difficulties with using the Zonda inhaler, ask your doctor, pharmacist, or nurse for help. If necessary, you can wipe the mouthpiece of the Zonda inhaler with a dry cloth or tissue. Do not breathe out into the Zonda inhaler. While using Braltus, be careful not to get the powder into your eyes, as it may cause blurred vision, eye pain, and/or eye redness; immediatelyrinse your eyes with warm water and immediatelyconsult your doctor for further advice. If you feel that your breathing difficulties are worsening, you should contact your doctor as soon as possible.
Use in children and adolescents
Braltus is not recommended for use in children and adolescents under 18 years of age.
Using more than the recommended dose of Braltus
If you inhale the contents of more than one Braltus capsule per day, you should immediatelycontact your doctor. You may be at increased risk of side effects, such as: dry mouth, constipation, difficulty urinating, rapid heart rate, or blurred vision.
Missing a dose of Braltus
If you miss a dose, take it as soon as possible, but nevertake two doses at the same time or on the same day. Take your next dose at the usual time. Do nottake a double dose to make up for a missed dose.
Stopping use of Braltus
Before stopping use of Braltus, consult your doctor or pharmacist. After stopping use of the medicine, your COPD symptoms may worsen. If you have any further questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. After using the medicine, severe allergic reactions, such as swelling of the face or throat (angioedema) or other hypersensitivity reactions (such as a sudden drop in blood pressure or dizziness) or wheezing or shortness of breath may occur alone or as part of a severe allergic reaction (anaphylaxis). Such serious side effects are rare. Additionally, as with other inhalation medicines, after inhaling the medicine, some patients may experience sudden chest tightness, coughing, wheezing, or shortness of breath (bronchospasm) immediately after use. If you experience any of these symptoms, you should immediatelycontact your doctor. You should consult your doctor before using Braltus again. If you experience wheezing or shortness of breath, you should use a fast-acting inhalation bronchodilator. Other side effects reported by people using this medicine are listed below by frequency:
Common: may affect up to 1 in 10 people
- dry mouth (usually mild)
Uncommon: may affect up to 1 in 100 people
- dizziness
- headache
- taste disturbances
- blurred vision
- irregular heartbeat (atrial fibrillation)
- throat irritation
- hoarseness (dysphonia)
- cough
- gastroesophageal reflux disease, which may cause heartburn
- constipation
- fungal infections of the mouth and throat (oral thrush)
- rash
- difficulty urinating (urinary retention)
- painful or difficult urination
Rare: may affect up to 1 in 1000 people
- difficulty sleeping (insomnia)
- seeing a rainbow-colored ring around a light source or changed color vision, along with eye redness (glaucoma)
- increased eye pressure
- irregular heartbeat (supraventricular tachycardia)
- rapid heart rate (tachycardia)
- palpitations
- chest tightness associated with coughing, wheezing, or shortness of breath occurring immediately after inhaling the medicine (bronchospasm)
- nasal bleeding
- laryngitis
- sinusitis
- intestinal obstruction (including paralytic ileus)
- gingivitis
- glossitis
- difficulty swallowing (dysphagia)
- stomatitis
- nausea
- hives
- itching
- urinary tract infections
Frequency cannot be estimated from the available data:
- dehydration
- tooth decay
- skin infections or ulcers
- dry skin
- joint swelling
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. You can also report side effects directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, e-mail: [email protected]. You can also report side effects to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Braltus
Keep the medicine out of the sight and reach of children. Do not use the medicine after the expiry date stated on the carton and on the label after EXP. The expiry date refers to the last day of that month. Do not store in the refrigerator or freeze. Keep the bottle tightly closed. Store in the original packaging to protect from moisture. Shelf life after first opening the bottle: 30 days (bottles containing 15 capsules) or 60 days (bottles containing 30 capsules). The Zonda inhaler should only be used with the capsules from the bottle supplied in the same packaging as the inhaler, or with the capsules from the bottle supplied in the packaging attached to the packaging with the inhaler. Do not reuse the inhaler for a second bottle of capsules. The Zonda inhaler should be discarded after 15 uses (if used with a bottle containing 15 capsules) or 30 uses (if used with a bottle containing 30 capsules). Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment. The inhaler should be disposed of in accordance with local regulations.
6. Contents of the packaging and other information
What Braltus contains
- The active substance is tiotropium. Each capsule contains 13 micrograms of tiotropium (as bromide). During inhalation, each capsule delivers 10 micrograms of tiotropium through the Zonda inhaler mouthpiece, which is then inhaled by the patient into the lungs.
- The other ingredients are: lactose monohydrate (capsule contents) and hypromellose (capsule shell)
What Braltus looks like and contents of the pack
Braltus 10 micrograms/delivered dose, powder for inhalation in a hard capsule is a colorless and transparent, hard capsule containing a white powder. The medicine is supplied in a bottle with a screw cap. The bottle, along with the Zonda inhaler, is packaged in a carton. The Zonda inhaler has a green body and a white button. Braltus is available in packs containing 15 or 30 capsules and a Zonda inhaler, and in bulk packs containing 60 capsules (2 packs of 30) and 2 Zonda inhalers, or 90 capsules (3 packs of 30) and 3 Zonda inhalers. Pack: 15 or 30 capsules (bottle) in a carton with 1 Zonda inhaler packaged in a separate carton. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer:
Marketing authorization holder
Teva Pharmaceuticals Polska Sp. z o.o., ul. Emilii Plater 53, 00-113 Warsaw, tel.: (22) 345 93 00
Manufacturer:
Laboratorios LICONSA S.A., Avda. Miralcampo, No 7, Polígono Industrial Miralcampo, 19200 Azuqueca de Henares, Guadalajara, Spain, Teva Pharma B.V., Swensweg 5, 2031 GA Haarlem, Netherlands, Actavis Ltd, BLB015, BLB016, Bulebel Industrial Estate, Zejtun, ZTN3000, Malta, Teva Operations Poland Sp. z o.o., ul. Mogilska 80, 31-546 Kraków, Poland
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria - Braltus 10 Mikrogramm pro abgegebener Dosis Kapseln mit Inhalationspulver, Belgium - Braltus 10 micrograms Inhalatiepoeder in harde capsule/ Poudre pour inhalation en gélule/ Hartkapsel mit Pulver zur Inhalation, Bulgaria - Braltus 10 микрограма прах за инхалация, твърда капсула, Croatia - Braltus 10 mikrograma po isporučenoj dozi, prašak inhalata, tvrde kapsule, Cyprus - Braltus 10 μικρογραμμάρια ανά χορηγούμενη δόση, κόνις για εισπνοή, καψάκιο, σκληρό, Czech Republic - Braltus 10 mikrogramů/dávka, prášek k inhalaci ve tvrdých tobolkách, Denmark - Braltus, Estonia - Braltus, Finland - Braltus 10 mikrog / vapautunut annos inhalaatiojauhe, kapseli, kova, Germany - Braltus 10 Mikrogramm Hartkapseln mit Pulver zur Inhalation, Greece - Braltus 10 μικρογραμμάρια ανά χορηγούμενη δόση, κόνις για εισπνοή, καψάκιο, σκληρό, Hungary - Braltus 10 mikrogramm adagolt inhalációs por kemény kapszulában, Ireland - Braltus 10 microgram per delivered dose inhalation powder, hard capsule, Italy - Tiotropio Teva Italia 10 microgrammi per dose erogata polvere per inalazione, capsula rigida, Latvia - Braltus 10 mikrogrami saņemtajā devā, inhalācijas pulveris cietās kapsulās, Lithuania - Braltus 10 mikrogramų/dozėje įkvepiamieji milteliai (kietoji kapsulė), Luxembourg - Braltus 10 Mikrogramm pro abgegebener Dosis Kapsel mit Inhalationspulver, Netherlands - Braltus 10 microgram per afgegeven dosis inhalatiepoeder in harde capsules, Norway - Braltus, Poland - Braltus, Portugal - Braltus, Romania - Braltus 10 micrograme pulbere de inhalat, capsula, Slovakia - Braltus 10 mikrogramov, Slovenia - Braltus 10 mikrogramov na dovedeni odmerek, prašek za inhaliranje, trde kapsule, Spain - Braltus 10 microgramos/dosis liberada polvo para inhalación, Sweden - Braltus, United Kingdom - Braltus 10 microgram per delivered dose inhalation powder, hard capsule
Date of last revision of the leaflet: June 2023
Other side of the leaflet:
Instructions for using the Zonda inhaler
Dear Patient, The Zonda inhaler allows you to take the medicine contained in the Braltus capsule, as prescribed by your doctor for breathing problems. You should carefully follow your doctor's instructions when using Braltus. The Zonda inhaler is designed only for Braltus capsules; do not use it to administer other medicines. Braltus capsules should only be taken using the Zonda inhaler. Do notuse any other inhaler to administer Braltus capsules. Each capsule contains a small amount of powder. Do notopen the capsules, as this may cause the inhaler to malfunction. The Zonda inhaler should only be used with the capsules from the bottle supplied in the same packaging as the inhaler, or with the capsules from the bottle supplied in the packaging attached to the packaging with the inhaler. Do not reuse the inhaler for a second bottle of capsules. The Zonda inhaler should be discarded after 15 uses (if used with a bottle containing 15 capsules) or 30 uses (if used with a bottle containing 30 capsules).
Zonda

- 1. Dust protective cap
- 2. Mouthpiece
- 3. Body
- 4. Piercing button
- 5. Central chamber
- 1. Open the dust protective cap by sliding it upwards.

- 2. Hold the body of the inhaler and open the mouthpiece by pulling it upwards, in the direction of the arrow.

- 3. Immediately before use, remove the Braltus capsule from the bottle and close the bottle tightly. Place one capsule in the central chamber of the inhaler body. Do notstore the capsule in the Zonda inhaler.

- 4. To avoid the risk of choking, NEVER insert the capsule directly into the mouthpiece.
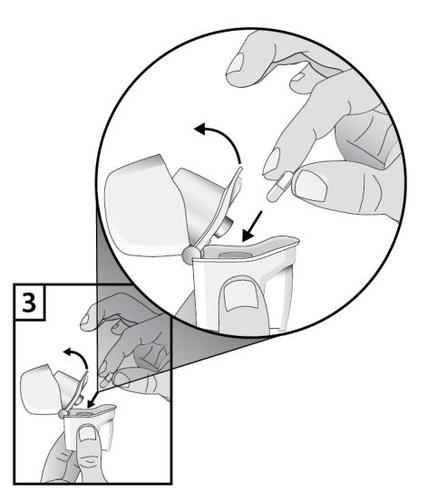
- 5. Close the mouthpiece until it clicks, and leave the dust protective cap open.

- 6. Holding the inhaler with the mouthpiece facing upwards, press the piercing button once fully, and then release it. The capsule will be pierced, and the medicine will be ready for you to inhale.

- 7. Exhale deeply. It is important to do this away from the mouthpiece. Never exhale into the mouthpiece.
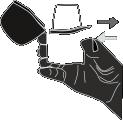
- 8. Place the mouthpiece in your mouth and hold your head in an upright position. Hold the mouthpiece between your lips and take a slow and deep breath in, so that you can hear or feel the vibration of the capsule in the central chamber. Then, hold your breath for as long as you can, while removing the inhaler from your mouth. Return to normal breathing. To ensure the capsule is empty, repeat steps 7 and 8.

- 9. After use, reopen the mouthpiece and remove the used capsule. Close the mouthpiece and dust protective cap, and put the Zonda inhaler away.
The Zonda inhaler is a medical device (CE) Manufacturer: Laboratorios LICONSA S.A., Avda. Miralcampo, No 7, Polígono Industrial Miralcampo, 19200 Azuqueca de Henares, Guadalajara, Spain, 0051


- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterActavis Ltd. Laboratorios Liconsa S.A. Teva Operations Poland Sp. z o.o. Teva Pharma B.V.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BraltusDosage form: Powder, 18 mcgActive substance: tiotropium bromideManufacturer: Ferrer Internacional, S.A.Prescription requiredDosage form: Powder, 18 mcg/measured doseActive substance: tiotropium bromidePrescription requiredDosage form: Powder, 18 mcg/measured doseActive substance: tiotropium bromidePrescription not required
Alternatives to Braltus in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Braltus in Ukraine
Alternative to Braltus in Spain
Online doctors for Braltus
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Braltus – subject to medical assessment and local rules.














