
Taptiqom

Ask a doctor about a prescription for Taptiqom

How to use Taptiqom
Leaflet accompanying the packaging: patient information
Taptiqom, 15 micrograms/ml + 5 mg/ml,
eye drops, solution in a single-dose container
Tafluprost + Timolol
Please read carefully the contents of the leaflet before using the medicine, as it contains
important information for the patient.
- Please keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, please consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Taptiqom and what is it used for
- 2. Important information before using Taptiqom
- 3. How to use Taptiqom
- 4. Possible side effects
- 5. How to store Taptiqom
- 6. Contents of the packaging and other information
1. What is Taptiqom and what is it used for
What type of medicine is it and how does it work?
Taptiqom eye drops contain tafluprost and timolol. Tafluprost belongs to a group of medicines called prostaglandin analogues, and timolol belongs to a group of medicines called beta-adrenergic blockers. Tafluprost and timolol work together to lower the pressure in the eye. Taptiqom is used when the pressure in the eye is too high.
What is this medicine used for?
Taptiqom is used to treat a type of glaucoma called open-angle glaucoma, and a condition called ocular hypertension in adults. Both of these conditions are associated with increased pressure within the eye and can lead to vision disturbances.
2. Important information before using Taptiqom
When not to use Taptiqom:
- if the patient is allergic to tafluprost, timolol, beta-adrenergic blockers, or any of the other ingredients of this medicine (listed in section 6);
- if the patient has a respiratory disease, such as asthma, severe chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, and/or prolonged coughing);
- if the patient has slow heart rate, heart failure, or irregular heart rhythm.
Warnings and precautions
Before starting to use Taptiqom, the patient should discuss it with their doctor, pharmacist, or nurse.
Before using this medicine, the patient should inform their doctor if they currently have or have had any of the following conditions:
- coronary heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, low blood pressure;
- irregular heart rhythm, such as slow heart rate;
- breathing difficulties, asthma, or chronic obstructive pulmonary disease;
- circulatory disorders (such as Raynaud's disease or Raynaud's syndrome);
- diabetes, as timolol may mask the symptoms of low blood sugar;
- overactive thyroid gland, as timolol may mask the symptoms of thyroid disease;
- any allergy or anaphylactic reaction;
- myasthenia (a rare disease that causes muscle weakness);
- other eye diseases, such as corneal disease (the transparent tissue covering the front of the eye) or eye disease requiring surgical treatment.
The patient should inform their doctor if they have:
- kidney problems,
- liver problems.
It should be consideredthat Taptiqom may cause the following effects, some of which may be permanent:
- Taptiqom may increase the length, thickness, color intensity, and/or number of eyelashes, and may cause abnormal hair growth on the eyelids.
- Taptiqom may cause darkening of the skin color around the eyes. The patient should wipe away any excess solution from the skin around the eye. This will help reduce the risk of skin darkening.
- Taptiqom may change the color of the iris (the colored part of the eye). If Taptiqom is used in only one eye, the color of the treated eye may change permanently and differ from the other eye.
- Taptiqom may cause hair growth in areas where the solution comes into repeated contact with the skin.
Before surgery, the patient should tell their doctor that they are using Taptiqom, as timolol may affect the action of certain medicines used during anesthesia.
Children and adolescents
Taptiqom is not recommended for children and adolescents under 18 years of age due to the lack of data on its efficacy and safety in this age group.
Taptiqom and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Other medicines may affect the action of Taptiqom, and Taptiqom may affect the action of other medicines.
In particular, the patient should tell their doctor if they are taking or plan to take any of the following medicines:
- other eye drops for glaucoma,
- medicines used to lower blood pressure,
- heart medicines,
- medicines used to treat diabetes,
- quinidine (a medicine used to treat heart diseases and some types of malaria),
- antidepressant medicines, such as fluoxetine and paroxetine.
If the patient is also using other eye medicines, they should leave a gap of at least 5 minutes between administering Taptiqom and the other medicine.
Contact lenses
The patient should remove their contact lenses before administering the drops and wait at least 15 minutes before putting them back in.
Pregnancy, breastfeeding, and fertility
If the patient may become pregnant, they must use an effective method of contraception during treatment with Taptiqom. Taptiqom should not be used during pregnancy. Taptiqom should not be used during breastfeeding. The patient should consult their doctor.
Driving and using machines
Certain side effects associated with the use of Taptiqom, such as blurred vision, may affect the ability to drive or operate machines. The patient should not drive or operate any machines until they feel well and their vision is clear.
Taptiqom contains phosphate buffer
This medicine contains approximately 0.04 mg of phosphates per drop, which corresponds to 1.3 mg/ml.
In patients with severe damage to the transparent, front part of the eye (cornea), phosphates may rarely cause corneal clouding during treatment due to calcium accumulation.
3. How to use Taptiqom
This medicine should always be used exactly as prescribed by the doctor or pharmacist. If the patient has any doubts, they should consult their doctor or pharmacist.
The recommended dose is one drop of Taptiqom in the eye(s) once a day. The patient should not use more drops or use the medicine more often than prescribed by their doctor. This may reduce the effectiveness of Taptiqom.
Taptiqom should be used in both eyes only if prescribed by the doctor. The patient should discard the single-dose container with any unused solution immediately after use.
For use as eye drops only. Do not swallow.
The patient should not allow the single-dose container to come into contact with the eye or the area around the eye. This could cause eye injury. It could also lead to contamination of the eye with bacteria and subsequent infection, which could lead to serious damage or even vision loss. To avoid possible contamination of the single-dose container, the patient should not allow the tip of the container to come into contact with any surface.
Instructions for use:
In case of opening a new sachet:
The patient should not use single-dose containers if the sachet is damaged. The patient should tear the sachet along the dotted line. The patient should write the date of opening the sachet in the space provided on the sachet.
Each time the patient uses Taptiqom:
- 1. Wash hands.
- 2. Remove the strip of containers from the sachet.
- 3. Separate one single-dose container from the strip.
- 4. Put the remaining containers back in the sachet and fold the edge to close the sachet.
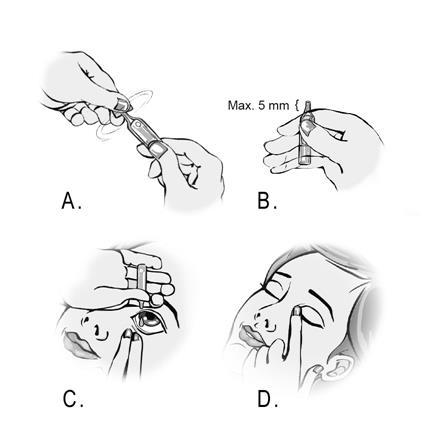
- 5. Open the container by twisting the tab at the top of the container. (Figure A)
- 6. Hold the container between the thumb and index finger. The patient should remember that the tip of the container should not protrude more than 5 mm beyond the edge of the index finger. (Figure B)
- 7. Tilt the head back or lie down. The patient should place their hand on their forehead. The index finger should be on the eyebrow line or resting against the bridge of the nose. The patient should look up. With the other hand, the patient should gently pull down the lower eyelid. The patient should not allow any part of the single-dose container to touch the eye or the area around the eye. The patient should gently squeeze the container to release one drop into the space between the lower eyelid and the eye. (Figure C)
- 8. Close the eye and press the inner corner of the eye with the finger for about two minutes. This will help prevent the drop from entering the tear duct. (Figure D)
- 9. Wipe away any excess solution from the skin around the eye.
If the drop does not get into the eye, the patient should try again.
If the doctor has prescribed drops for both eyes, the patient should repeat steps 7 to 9 for the second eye.
The contents of one single-dose container are sufficient for both eyes. The patient should discard the container with any unused solution immediately after use.
If the patient is also using other eye medicines, they should leave a gap of at least 5 minutes between administering Taptiqom and the other medicine.
In case of using more than the recommended dose of Taptiqom, the patient may experience dizziness, headache, heart symptoms, or breathing difficulties. If necessary, the patient should consult their doctor.
If the medicine is accidentally swallowed, the patient should contact their doctor.
In case of missing a dose of Taptiqom, the patient should take a single drop as soon as they remember, and then continue using the medicine as planned. However, if it is almost time for the next dose, the patient should not take the missed dose. The patient should not take a double dose to make up for the missed dose.
The patient should not stop using Taptiqom without consulting their doctor.If the patient stops using Taptiqom, the pressure in the eye will increase again. This may cause permanent damage to the eye.
In case of any further doubts about using this medicine, the patient should consult their doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Taptiqom can cause side effects, although not everybody gets them. Most side effects are not serious.
Usually, the patient can continue using the eye drops if the side effects are not serious. If the patient is concerned, they should talk to their doctor or pharmacist.
The following are known side effects of Taptiqom:
Common side effects
The following effects may occur in up to 1 in 10 patients:
Eyelid disorders
itching of the eye, irritation of the eye, eye pain, redness of the eye, changes in the length, thickness, and number of eyelashes, feeling of a foreign body in the eye, change in eyelash color, sensitivity to light, blurred vision.
Uncommon side effects
The following effects may occur in up to 1 in 100 patients:
Nervous system disorders
headache
Eyelid disorders
dry eye, redness of the eyelids, small pinpoint areas of inflammation on the surface of the eye, tearing, swelling of the eyelids, eye fatigue, eyelid inflammation, inflammation of the inside of the eye, feeling of discomfort in the eye, eye allergy, eye inflammation, abnormal sensations in the eye.
The following side effects have been observed with the use of the active substances of Taptiqom (tafluprost and timolol), and may also occur with the use of Taptiqom:
The following side effects have been observed with the use of tafluprost:
Eyelid disorders
reduced ability to see details, change in iris color (may be permanent), change in skin color around the eyes, swelling of the conjunctiva, discharge from the eye, discoloration of the conjunctiva, nodules on the conjunctiva, sunken eye, inflammation of the iris (the colored part of the eye)/inflammation of the uvea (the vascular layer of the eye).
Macular edema (swelling of the macula, the part of the retina responsible for central vision, which can lead to vision impairment).
Skin disorders
abnormal hair growth on the eyelids.
Respiratory disorders
worsening of asthma, shortness of breath.
The following side effects have been observed with the use of timolol:
Immune system disorders
allergic reactions, including angioedema, rash, and urticaria, severe life-threatening allergic reaction, itching.
Metabolic and nutritional disorders
low blood sugar
Psychiatric disorders
depression, difficulty sleeping, nightmares, memory loss, nervousness, hallucinations.
Nervous system disorders
dizziness, fainting, unusual sensations (such as tingling and numbness), worsening of myasthenia symptoms (muscle disorders), stroke, decreased blood flow to the brain.
Eyelid disorders
inflammation of the cornea, decreased corneal sensitivity, visual disturbances, including changes in refraction (sometimes caused by discontinuation of medicines that constrict the pupils), drooping of the upper eyelid, double vision, blurred vision, and detachment of the layer behind the retina with blood vessels after surgical treatment of trabeculectomy, which can cause vision disturbances, corneal ulcers.
Ear disorders
ringing in the ears.
Cardiac disorders
slow heart rate, chest pain, palpitations, swelling (fluid accumulation), changes in heart rhythm or rate, congestive heart failure (heart disease that can cause shortness of breath and swelling of the feet and ankles due to fluid accumulation), certain types of irregular heart rhythm, heart attack, heart failure.
Vascular disorders
low blood pressure, cold extremities, Raynaud's phenomenon, cold hands and feet.
Respiratory disorders
constriction of airways in the lungs (mainly in patients with pre-existing disease), difficulty breathing, cough.
Gastrointestinal disorders
nausea, indigestion, diarrhea, dry mouth, taste disturbances, abdominal pain, vomiting.
Skin disorders
hair loss, skin rash with a white-silver color (psoriasis-like rash) or exacerbation of psoriasis, skin rash.
Musculoskeletal disorders
muscle pain not caused by physical exertion, joint pain.
Reproductive system and breast disorders
Peyronie's disease (which can cause curvature of the penis), sexual dysfunction, decreased sexual desire.
General disorders
muscle weakness/fatigue, thirst.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, it is possible to gather more information on the safety of the medicine.
5. How to store Taptiqom
The medicine should be stored out of sight and reach of children.
The patient should not use this medicine after the expiry date stated on the single-dose container, sachet, and carton after "EXP". The expiry date refers to the last day of the month.
Unopened sachets should be stored in the refrigerator (2°C – 8°C). The sachet should be opened just before using the eye drops, as unused containers from an opened sachet should be discarded after 28 days from the first opening of the sachet.
After the first opening of the sachet:
- Store the single-dose container in the sachet to protect it from light.
- Do not store above 25°C.
- Discard unused single-dose containers after 28 days from the first opening of the sachet.
- Discard the opened single-dose container with any unused solution immediately after use.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What does Taptiqom contain
- The active substances of Taptiqom are tafluprost and timolol. 1 ml of solution contains 15 micrograms of tafluprost and 5 mg of timolol.
- The other ingredients are: glycerol, disodium phosphate dodecahydrate, disodium edetate, polysorbate 80, hydrochloric acid, and sodium hydroxide (to adjust pH) and water for injections.
What does Taptiqom look like and what does the packaging contain
Taptiqom is a clear, colorless solution, supplied in plastic single-dose containers containing 0.3 ml of solution. One sachet contains ten single-dose containers. Taptiqom is available in packs of 30 or 90 single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Taptiqom: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom (Northern Ireland).
Loyada: Italy
Date of last revision of the leaflet: 06/2022
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSanten OY
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TaptiqomDosage form: Drops, 50 mcg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Pharmaselect International Beteiligungs GmbHPrescription not requiredDosage form: Drops, (0.3 mg + 5 mg)/mlActive substance: timolol, combinationsPrescription requiredDosage form: Drops, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Genetic S.p.APrescription required
Alternatives to Taptiqom in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Taptiqom in Spain
Alternative to Taptiqom in Ukraine
Online doctors for Taptiqom
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Taptiqom – subject to medical assessment and local rules.














