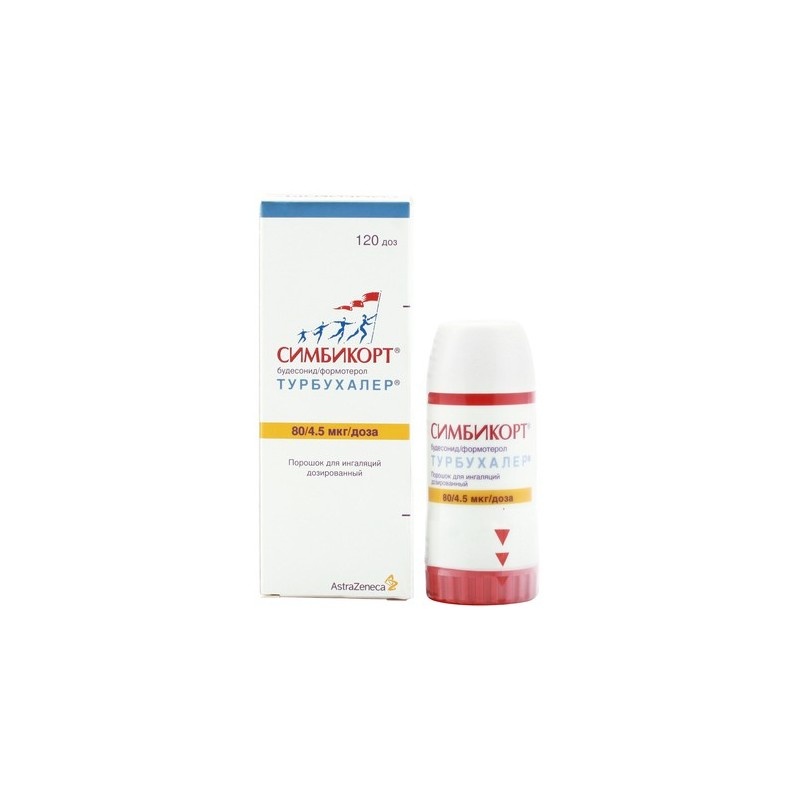
Simbicort Turbuhaler

Ask a doctor about a prescription for Simbicort Turbuhaler

How to use Simbicort Turbuhaler
Patient Information Leaflet: User Information
Symbicort Turbuhaler, (320 µg + 9 µg)/inhalation dose, inhalation powder
Budesonide + Formoterol fumarate dihydrate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet:
- 1. What is Symbicort Turbuhaler and what is it used for
- 2. Important information before using Symbicort Turbuhaler
- 3. How to use Symbicort Turbuhaler
- 4. Possible side effects
- 5. How to store Symbicort Turbuhaler
- 6. Contents of the pack and other information
1. What is Symbicort Turbuhaler and what is it used for
Symbicort Turbuhaler is an inhaler medicine used to treat asthma in adults and adolescents from 12 to 17 years old. It is also used to treat symptoms of chronic obstructive pulmonary disease (COPD) in adults aged 18 and over. It contains two different active substances: budesonide and formoterol fumarate dihydrate.
- Budesonide belongs to a group of medicines called corticosteroids. The medicine works by reducing and preventing swelling and inflammation in the lungs.
- Formoterol fumarate dihydrate belongs to a group of medicines called long-acting beta2-adrenergic receptor agonists or bronchodilators. Its action is to relax the muscles in the airways, making it easier to breathe.
Asthma
In the case of asthma, the doctor will prescribe the use of two inhaler medicines: Symbicort Turbuhaler and another medicine for occasional use.
- Symbicort Turbuhaler is used daily. Its use helps prevent the occurrence of asthma symptoms.
- The inhaler medicine for occasional use should be used when asthma symptoms occur to facilitate breathing.
Symbicort Turbuhaler should not be used occasionally when symptoms occur.
Chronic Obstructive Pulmonary Disease (COPD)
Symbicort Turbuhaler can also be used to treat symptoms of COPD in adults. COPD is a chronic respiratory disease in the lungs, which is often caused by smoking.
2. Important information before using Symbicort Turbuhaler
When not to use Symbicort Turbuhaler:
Warnings and precautions
Before starting to use Symbicort Turbuhaler, talk to your doctor or pharmacist if you have:
- diabetes.
- lung infection.
- high blood pressure or if you have ever had heart problems (including irregular heartbeat, very fast heart rate, narrowing of the arteries or heart failure).
- thyroid or adrenal gland problems.
- low potassium levels in the blood.
- severe liver problems. If you experience blurred vision or other vision problems, you should contact your doctor.
Symbicort Turbuhaler and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as any medicines you plan to take.
Especially, inform your doctor or pharmacist if you are taking any of the following medicines:
- beta-blockers (such as atenolol or propranolol, used to treat high blood pressure), also in the form of eye drops (such as timolol used to treat glaucoma).
- medicines used in the case of accelerated or irregular heart rhythm (such as quinidine).
- medicines, such as digoxin, often used to treat heart failure.
- diuretics (such as furosemide), used to treat high blood pressure.
- oral steroids (such as prednisolone).
- xanthine derivatives (e.g. theophylline or aminophylline). These are medicines often used to treat asthma.
- other bronchodilators (such as salbutamol).
- tricyclic antidepressants (such as amitriptyline) and the antidepressant nefazodone.
- phenothiazine medicines (such as chlorpromazine and prochlorperazine).
- HIV protease inhibitors (e.g. ritonavir) used to treat HIV infection.
- medicines used in infections (such as ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin and telithromycin).
- medicines used in Parkinson's disease (e.g. levodopa).
- medicines used in thyroid diseases (e.g. levothyroxine).
If you are taking any of these medicines or have any doubts about taking other medicines, you should contact your doctor or pharmacist before using Symbicort Turbuhaler.
You should also inform your doctor or pharmacist if you are planning to undergo general anesthesia for a surgical or dental procedure.
Pregnancy, breastfeeding and fertility
- If you are pregnant or planning to become pregnant, you should tell your doctor before using Symbicort Turbuhaler; do not use Symbicort Turbuhaler without your doctor's recommendation.
- If you become pregnant while taking Symbicort Turbuhaler, do not stop taking Symbicort Turbuhaler, but contact your doctor immediately.
- A breastfeeding woman should consult her doctor before starting to use Symbicort Turbuhaler.
Driving and using machines
Symbicort Turbuhaler has no or negligible influence on the ability to drive and use machines.
Symbicort Turbuhaler contains lactose
Symbicort Turbuhaler contains lactose (a type of sugar). If you have been told that you have an intolerance to some sugars, you should talk to your doctor before taking this medicine. The amount of lactose in one dose of this medicine is usually not enough to cause problems in people with lactose intolerance.
The lactose excipient contains small amounts of milk proteins, which may cause allergic reactions.
3. How to use Symbicort Turbuhaler
- This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
- It is important to use Symbicort Turbuhaler every day, even if you do not have symptoms of asthma or COPD at the time.
- If Symbicort Turbuhaler is used to treat asthma, your doctor will regularly check the severity of your asthma symptoms.
If you are taking oral steroids for asthma or COPD, when introducing Symbicort Turbuhaler, your doctor may reduce the number of oral steroid tablets you take. If you have been taking oral steroids in tablet form for a long time, your doctor may occasionally recommend blood tests. After reducing the dose of oral steroids, you may feel worse, and your respiratory symptoms may worsen. Symptoms such as a stuffy nose, runny nose, weakness or muscle or joint pain and rash (rash) may occur. If any of these symptoms worry you or if you experience symptoms such as headache, fatigue, nausea or vomiting, you should contact your doctor immediately. It may be necessary to use other medicines if symptoms of allergy or arthritis occur. If you have any doubts about continuing to use Symbicort Turbuhaler, you should consult your doctor.
Your doctor may consider adding oral steroids to your usual treatment during periods of stress (e.g. chest infection or before surgery).
Important information about asthma or COPD symptoms
If you experience shortness of breath or wheezing while using Symbicort Turbuhaler, continue to use Symbicort Turbuhaler, but contact your doctor as soon as possible, as additional treatment may be necessary.
You should contact your doctor immediately if:
- you have difficulty breathing or wake up at night with an asthma attack.
- you have a feeling of tightness in the chest in the morning after getting up or the feeling of tightness in the chest lasts longer than usual. These symptoms may indicate inadequate control of asthma or COPD and may require immediate use of other or additional treatment.
Asthma
Use Symbicort Turbuhaler every day.Using Symbicort Turbuhaler helps prevent the occurrence of asthma symptoms.
Adults (18 years and older)
- The usual dose is 1 inhalation twice a day.
- Your doctor may increase the dose to 2 inhalations twice a day.
- If your symptoms are well controlled, your doctor may recommend taking the medicine once a day.
Adolescents (12-17 years old)
- The usual dose is 1 inhalation twice a day.
- If your symptoms are well controlled, your doctor may recommend taking the medicine once a day.
For children aged 6-11, a lower strength Symbicort Turbuhaler is available.
Symbicort Turbuhaler is not recommended for use in children under 6 years of age.
During treatment, your doctor will help you with the proper treatment of asthma. Your doctor will determine the smallest dose that will control your asthma symptoms. Do not change the dose of Symbicort Turbuhaler without consulting your doctor.
In case of asthma symptoms, use an additional inhaler for
occasional use.You should always carry an additional inhaler for occasional use with you. Do not use Symbicort Turbuhaler to treat asthma symptoms – use an additional inhaler for occasional use for this purpose.
Chronic Obstructive Pulmonary Disease (COPD)
- For use only in adults (18 years and older).
- The usual dose is 1 inhalation twice a day.
For patients with COPD, your doctor may also prescribe other bronchodilators, such as anticholinergic medicines (e.g. tiotropium bromide and ipratropium bromide).
Preparing the Symbicort Turbuhaler inhaler for first use
Before firstuse of a newSymbicort Turbuhaler inhaler, prepare it for use as follows:
- Remove the cap from the inhaler. When unscrewing, you may hear a characteristic rattling sound.
- Hold the Symbicort Turbuhaler inhaler upright, with the red wheel at the bottom.
- Turn the red wheel one full turn until it clicks. Then turn it one full turn in the opposite direction (the direction you start turning the wheel does not matter). You will hear a characteristic click. It does not matter whether the click occurs after the first or second turn.
- Repeat the above steps, turning the red wheel in both directions.
- The Symbicort Turbuhaler inhaler is now loaded and ready for use.
Using the inhaler
To perform an inhalation, follow the steps below each time.
- 1. Remove the cap from the inhaler. When unscrewing, you may hear a characteristic rattling sound.
- 2. Hold the Symbicort Turbuhaler inhaler upright,with the red wheel at the bottom.
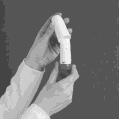
- 3. When loading the medicine, do not hold the Symbicort Turbuhaler inhaler by the mouthpiece. To load a dose of medicine, turn the red wheel until it clicks in one direction.
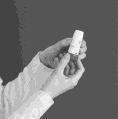
Then turn the wheel until it clicks in the opposite direction (the direction you start turning the wheel does not matter). You will hear a characteristic click. It does not matter whether the click occurs after the first or second turn.
The Symbicort Turbuhaler inhaler is now loaded and ready for use. Only load a dose of medicine immediately before use.
- 4. Hold the Symbicort Turbuhaler inhaler away from your mouth. Perform a gentle exhalation (without effort). Do not exhale through the Symbicort Turbuhaler inhaler mouthpiece.
- 5. Place the mouthpiece of the inhaler gently between your teeth. Close your mouth (hold the mouthpiece with your lips) and perform a maximum deep and maximum intense inhalation through your mouth. Do not chew or bite the mouthpiece.
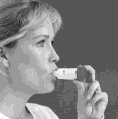
- 6. Remove the Symbicort Turbuhaler inhaler from your mouth. Perform a calm exhalation.The amount of medicine in one inhalation dose is very small, so after inhalation, the taste of the medicine may not be noticeable. However, if you follow the instructions, you can be sure that the dose of medicine has been taken and the medicine is in your lungs.
- 7. If a second inhalation is recommended, repeat the steps described in points 2 to 6.
- 8. After use, put the cap back on the inhaler and close it tightly.

- 9. Rinse your mouth with water after taking a dose of medicine in the morning and (or) evening. Do not swallow the water. Do not remove or turn the mouthpiece. It is connected to the Symbicort Turbuhaler inhaler and cannot be removed. Do not use the Symbicort Turbuhaler inhaler if it is damaged or if the mouthpiece is not attached to the inhaler.
As with all inhalers, caregivers should ensure that children who have been prescribed Symbicort Turbuhaler use the correct inhalation technique, as described above.
Cleaning the Symbicort Turbuhaler inhaler
Wipe the outer surface of the mouthpiece with a dry cloth once a week. Do not use water or other liquids.
When to start using a new inhaler
- The dose counter window shows how many doses (inhalations) are left in the Symbicort Turbuhaler inhaler; a full inhaler contains 60 doses of medicine.

- The dose counter scale is marked every 10 inhalations, so the counter will not show a change after a single dose of medicine.
- The appearance of a red mark on the edge of the dose counter window means that there are about 20 doses of medicine left in the inhaler. When administering the last 10 doses of medicine, the background of the dose counter window is red. When the number 0 on the red background is in the middle of the dose counter window, it means that you should start using a new Symbicort Turbuhaler inhaler.
Note:
- After the inhaler is empty, the wheel will still turn and make a characteristic clicking sound.
- The sound heard when shaking the Symbicort Turbuhaler inhaler is made by the desiccant, not the medicine. Therefore, you cannot assess the contents of the Symbicort Turbuhaler inhaler based on the sound it makes when shaken.
- If more than one dose of medicine is loaded by mistake, the patient will still receive only one dose of medicine. However, all loaded doses will be recorded in the dose counter.
Using a higher than recommended dose of Symbicort Turbuhaler
It is important that you take the doses described in the leaflet or recommended by your doctor.
Do not increase the recommended dose without consulting your doctor.
The most common symptoms that may occur after taking a higher than recommended dose of Symbicort Turbuhaler are: tremors, headaches and rapid heart rate.
Missing a dose of Symbicort Turbuhaler
- If you miss a dose, take it as soon as you remember. However, if it is almost time for your next dose, skip the missed dose.
- Do nottake a double dose to make up for a missed dose.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Symbicort Turbuhaler and contact your doctor immediately if you experience any of the following symptoms:
- swelling of the face, especially around the mouth (tongue and/or throat and/or difficulty swallowing) or hives occurring at the same time as difficulty breathing (angioedema) and/or sudden feeling of weakness (fainting). This may indicate an allergic reaction. It occurs rarely, in less than 1 in 1000 patients.
- Sudden worsening of wheezing and shortness of breath occurring immediately after inhalation of the medicine.
If you experience any of these symptoms, stop using Symbicort Turbuhaler and use your relief inhaler.
Contact your doctor immediatelyas you may need to change your treatment.
Such symptoms occur very rarely, in less than 1 in 10,000 patients.
Other possible side effects:
Common (may affect up to 1 in 10 people)
- Palpitations (feeling of heartbeat), tremors. If these symptoms occur, they are usually mild and usually disappear during continued use of Symbicort Turbuhaler.
- Thrush (fungal infection) in the mouth. The occurrence of thrush is less likely if you rinse your mouth with water after using Symbicort Turbuhaler.
- Mild throat irritation, cough and hoarseness.
- Headache.
- Pneumonia (lung infection) in patients with COPD. You should tell your doctor if any of the following symptoms occur while using Symbicort Turbuhaler; they may be symptoms of a lung infection:
- Fever or chills.
- Increased production of mucus, change in mucus color.
- Worsening cough or increased breathing difficulties.
Uncommon (may affect up to 1 in 100 people)
- Anxiety, nervousness or restlessness.
- Sleep disturbances.
- Dizziness.
- Nausea (nausea).
- Rapid heart rate.
- Bruises on the skin.
- Muscle cramps.
- Blurred vision.
Rare (may affect up to 1 in 1000 people)
- Rash, itching.
- Bronchospasm (bronchial muscle spasm manifesting as wheezing). If wheezing occurs immediately after using the medicine, stop using Symbicort Turbuhaler and contact your doctor immediately.
- Low potassium levels in the blood.
- Irregular heartbeat.
Very rare (may affect up to 1 in 10,000 people)
- Depression.
- Changes in behavior, especially in children.
- Chest pain or feeling of tightness in the chest (symptoms of angina pectoris).
- Increased blood sugar (glucose) levels.
- Taste disturbances, such as unpleasant taste in the mouth.
- Changes in blood pressure.
Inhaled corticosteroids may affect the production of steroid hormones in the body, especially if they are used for a long time in high doses. The symptoms include:
- changes in bone mineral density (osteoporosis)
- cataract (clouding of the lens in the eye)
- glaucoma (increased pressure in the eye)
- slowed growth in children and adolescents
- effects on the adrenal glands (small glands located near the kidneys).
The likelihood of these symptoms is much lower when using inhaled corticosteroids than when taking corticosteroids in tablets.
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Symbicort Turbuhaler
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the carton or on the label of the inhaler after EXP. The expiry date refers to the last day of the month stated.
- There are no special storage instructions for this medicine.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Symbicort Turbuhaler contains
The active substances of the medicine are budesonide and formoterol fumarate dihydrate. Each inhalation dose of Symbicort Turbuhaler contains 320 micrograms of budesonide and 9 micrograms of formoterol fumarate dihydrate.
The other ingredient of the medicine is lactose monohydrate (which contains milk proteins).
What Symbicort Turbuhaler looks like and contents of the pack
Symbicort Turbuhaler is an inhaler medicine. The inhalation powder is white. Each inhaler contains 60 doses of medicine. The inhaler has a white body and a red wheel. The wheel has a Braille code with the number 6, which allows identification and distinguishes the medicine from other inhaler medicines from AstraZeneca.
Symbicort Turbuhaler is available in packs containing 1, 2, 3, 10 or 18 inhalers, each containing 60 doses.
Not all pack sizes may be marketed.
Marketing authorization holder
AstraZeneca AB
151 85 Södertälje
Sweden
Manufacturer
AstraZeneca AB
Forskargatan 18
151 36 Södertälje
Sweden
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Country | Proprietary name and strength |
| Austria | Symbicort forte Turbohaler 320 μg/9 μg/inhalation |
| Belgium | Symbicort forte Turbohaler 320 μg/ 9μg/inhalatie |
| Bulgaria | Symbicort Turbuhaler 320 μg/9 μg/inhalation |
| Croatia | Symbicort Turbuhaler 320 μg/9 μg/inhalation |
| Cyprus | Symbicort Turbuhaler 320 μg/9 μg/inhalation |
| Czech Republic | Symbicort Turbuhaler 320 μg/9 μg/inhalaci |
| Denmark | Symbicort Forte Turbuhaler 320 μg/9 μg/inhalation |
| Estonia | Symbicort Turbuhaler, 320 μg/9 μg |
| Finland | Symbicort Turbuhaler 320 μg/9 μg/inhalation |
| France | Symbicort Turbuhaler 400 μg/12 μg/inhalation |
| Germany | Symbicort Turbuhaler 320 μg/9 μg/inhalation |
| Greece | SYMBICORT TURBUHALER 320 μg/9 μg/inhalation |
| Hungary | Symbicort forte Turbuhaler 320 μg/9 μg/inhalacios |
| Iceland | Symbicort forte Turbuhaler 320 μg/9 μg/inhalation |
| Ireland | Symbicort Turbuhaler 400 μg/12 μg/inhalation |
| Italy | SYMBICORT 320 μg/9 μg/inhalazione |
| Latvia | Symbicort forte Turbuhaler 320 μg/9 μg/inhalacija |
| Lithuania | Symbicort Turbuhaler 320 μg/9 μg/dose |
| Luxembourg | Symbicort forte Turbohaler 320 μg/9 μg/inhalatiojn |
| Malta | Symbicort Turbuhaler 400 μg/12 μg/inhalation |
| Netherlands | Symbicort Turbuhaler 400 μg/12 μg/inhalation |
| Norway | Symbicort forte Turbuhaler 320μg/9μg/inhalasjon |
| Poland | Symbicort Turbuhaler 320 μg/9 μg/inhalation dose |
| Portugal | Symbicort Turbohaler 320 μg/9 μg/inhalacao |
| Romania | Symbicort Turbuhaler 320 μg/9 μg/inhalat |
| Slovakia | Symbicort Turbuhaler forte 400 μg/12 μg/inhalacia |
| Slovenia | Symbicort forte Turbuhaler 320 μg/9 μg/ inhaliranje |
| Spain | Symbicort forte Turbuhaler 320 μg/9 μg/inhalacion |
| Sweden | Symbicort forte Turbuhaler 320 μg/9 μg/inhalation |
| United Kingdom | Symbicort Turbohaler 400 μg/12 μg/inhalation |
For more detailed information, please contact the representative of the marketing authorization holder:
AstraZeneca Pharma Poland Sp. z o.o.
ul. Postępu 14
02-676 Warsaw
tel.: +48 22 245 73 00
fax: +48 22 485 30 07
Date of last revision of the leaflet:October 2023
Detailed and up-to-date information about this product is available by scanning the QR code on the Patient Information Leaflet and on the outer packaging of the medicine. The same information is also available on the website: www.turbuhaler.pl
QR code area
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAstraZeneca AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Simbicort TurbuhalerDosage form: Powder, (160 mcg + 4.5 mcg)/inh. doseActive substance: formoterol and budesonidePrescription requiredDosage form: Powder, (320 mcg + 9 mcg)/inh. doseActive substance: formoterol and budesonideManufacturer: Aeropharm GmbH Lek farmacevtska družba d.d. (Lek Pharmaceuticals d.d.) Salutas Pharma GmbHPrescription requiredDosage form: Powder, (160 mcg + 4.5 mcg)/inh. doseActive substance: formoterol and budesonideManufacturer: Orion CorporationPrescription required
Alternatives to Simbicort Turbuhaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Simbicort Turbuhaler in Ukraine
Alternative to Simbicort Turbuhaler in Spain
Online doctors for Simbicort Turbuhaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Simbicort Turbuhaler – subject to medical assessment and local rules.