Airbufo Forspiro

Ask a doctor about a prescription for Airbufo Forspiro

How to use Airbufo Forspiro
Leaflet accompanying the packaging: information for the user
Airbufo Forspiro, (320 micrograms + 9 micrograms)/inhalation dose,
inhalation powder, divided
Budesonide + Formoterol fumarate dihydrate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Airbufo Forspiro and what is it used for
- 2. Important information before using Airbufo Forspiro
- 3. How to use Airbufo Forspiro
- 4. Possible side effects
- 5. How to store Airbufo Forspiro
- 6. Contents of the packaging and other information
1. What is Airbufo Forspiro and what is it used for
Airbufo Forspiro is an inhaler medicine used to treat asthma in adults and adolescents aged 12 to 17 years. It is also used to treat symptoms of chronic obstructive pulmonary disease (COPD) in adults aged 18 years and older.
Airbufo Forspiro contains two medicines: budesonide and formoterol fumarate dihydrate.
- Budesonide belongs to a group of medicines called corticosteroids. It works by reducing and preventing swelling and inflammation in the lungs.
- Formoterol fumarate dihydrate belongs to a group of medicines called long-acting beta-2-adrenergic receptor agonists or bronchodilators. Its action is to relax the muscles in the airways. The medicine makes it easier to breathe.
Asthma
For the treatment of asthma, the doctor prescribes two inhaler medicines: Airbufo Forspiro and a separate medicine for emergency use.
- Airbufo Forspiro is used daily. The medicine helps prevent the onset of asthma symptoms.
- The emergency inhaler is used when asthma symptoms occur, to make breathing easier. Do not use Airbufo Forspiro, (320 micrograms + 9 micrograms)/inhalation dose, as an emergency inhaler.
Chronic Obstructive Pulmonary Disease (COPD)
Airbufo Forspiro can also be used in adults to treat symptoms of COPD. COPD is a chronic respiratory disease in the lungs, often caused by smoking.
2. Important information before using Airbufo Forspiro
When not to use Airbufo Forspiro:
- if the patient is allergic to budesonide, formoterol or any other ingredient of this medicine
(listed in section 6).
Warnings and precautions
Before starting to use Airbufo Forspiro, the patient should discuss it with their doctor or pharmacist if they:
- have diabetes,
- have a lung infection,
- have high blood pressure or have ever had heart disease (including irregular heartbeat, very fast heart rate, narrowed arteries, or heart failure),
- have thyroid or adrenal gland disease,
- have low potassium levels in the blood,
- have severe liver function disorders.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Airbufo Forspiro and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
They should especially tell their doctor or pharmacist if they are taking any of the following medicines:
- beta-blockers (such as atenolol or propranolol used to treat high blood pressure), also in eye drops (such as timolol used to treat glaucoma);
- medicines used to treat rapid or irregular heartbeat (such as quinidine, disopyramide, procainamide);
- medicines used to treat allergies, so-called antihistamines (e.g. terfenadine);
- oxytocin (a medicine used to accelerate childbirth);
- procarbazine (a medicine used to treat cancer);
- medicines such as digoxin, often used to treat heart failure;
- diuretics (such as furosemide) used to treat high blood pressure;
- corticosteroids (such as prednisolone) used to treat inflammation or prevent rejection of a transplanted organ;
- xanthine derivatives (such as theophylline or aminophylline) often used to treat asthma;
- other medicines used to dilate the airways (such as salbutamol);
- medicines used to treat depression, so-called tricyclic antidepressants (such as amitriptyline) and the antidepressant nefazodone;
- medicines used to treat mental illnesses, nausea or vomiting, so-called phenothiazines (such as chlorpromazine and prochlorperazine);
- medicines used to treat fungal or bacterial infections (such as ketoconazole, itraconazole, voriconazole, posaconazole) and (such as clarithromycin, telithromycin and furazolidone);
- medicines used to treat Parkinson's disease (such as levodopa);
- medicines used to treat thyroid diseases (such as levothyroxine);
- medicines called HIV protease inhibitors (such as ritonavir, cobicistat), used to treat HIV infection. The effect of Airbufo Forspiro may be enhanced and the doctor may need to closely monitor the patient's condition.
If any of these situations apply to the patient or they are not sure if they are taking any of these medicines, they should consult their doctor or pharmacist before using Airbufo Forspiro.
They should also tell their doctor or pharmacist if they are planning to have general anesthesia for an operation or dental procedure.
Pregnancy and breastfeeding
- If the patient is pregnant or plans to have a child, they should consult their doctor before using Airbufo Forspiro. They should not use Airbufo Forspiro during pregnancy, unless their doctor has advised them to do so.
- If the patient becomes pregnant while using Airbufo Forspiro, they should not stop using it, but should contact their doctor immediately.
- If the patient is breastfeeding, they should consult their doctor before using Airbufo Forspiro.
Driving and using machines
Airbufo Forspiro has no or negligible influence on the ability to drive and use machines.
Airbufo Forspiro contains lactose
Airbufo Forspiro contains lactose, a type of sugar. If the patient has been diagnosed with intolerance to some sugars, they should consult their doctor before taking the medicine. The amount of lactose in this medicine should not normally be a problem for people with lactose intolerance. Lactose, an excipient of the medicine, contains small amounts of milk proteins, which may cause allergic reactions.
3. How to use Airbufo Forspiro
- This medicine should always be used as directed by the doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
- It is important to use Airbufo Forspiro every day, even if the patient does not have symptoms of asthma or COPD at that time.
- If the patient is using Airbufo Forspiro to treat asthma, their doctor will regularly check their symptoms.
If the patient is taking steroid tablets to treat asthma or COPD, their doctor may reduce the number of tablets after starting to use Airbufo Forspiro. If the patient has been taking oral steroids for a long time, their doctor may occasionally recommend blood tests. After reducing the dose of oral steroids, the patient's general condition may be poor, even if their respiratory symptoms improve. They may experience symptoms such as a stuffy nose or discharge from the nose, weakness or muscle or joint pain, and rash (hives). If any of these symptoms worry the patient or they experience symptoms such as headache, fatigue, nausea or vomiting, they should contact their doctor immediately. If symptoms of an allergic reaction or arthritis occur, it may be necessary to use other medicines. If the patient is unsure whether to continue using Airbufo Forspiro, they should consult their doctor.
In periods of stress (e.g. associated with a chest infection or before surgery), the doctor may consider adding steroid tablets to the usual treatment.
Important information about asthma or COPD symptoms
If the patient experiences shortness of breath or wheezing while using Airbufo Forspiro, they should continue to use Airbufo Forspiro and consult their doctor as soon as possible, as additional treatment may be necessary.
The patient should consult their doctor immediately if:
breathing difficulties worsen or asthma symptoms wake them up often at night;
they experience chest tightness in the morning or chest tightness that lasts longer than usual.
These symptoms may indicate inadequate control of asthma or COPD and may require immediate use of other or additional treatment.
Asthma
Airbufo Forspiro should be used every day.This helps prevent the onset of asthma symptoms.
Adults (aged 18 years and older)
The usual dose is 1 inhalation twice a day.
The doctor may increase this dose to 2 inhalations twice a day.
After achieving control of symptoms, the doctor may recommend using the medicine once a day.
Adolescents (aged 12 to 17 years)
The usual dose is 1 inhalation twice a day.
After achieving control of symptoms, the doctor may recommend using the medicine once a day.
Airbufo Forspiro should not be used in children under 12 years of age.
The doctor will help the patient properly control their asthma symptoms and determine the smallest dose that will control the disease. The patient should not change the dose of the medicine without first discussing it with their doctor.
For the treatment of asthma symptoms when they occur, a separate "emergency inhaler" should be used.
This inhaler should always be carried with themso that it can be used if needed. The patient should not use Airbufo Forspiro (320 micrograms + 9 micrograms)/inhalation dose to treat asthma symptoms - this is what the emergency inhaler is for.
Chronic Obstructive Pulmonary Disease (COPD)
- The medicine should only be used in adults (aged 18 years and older).
- The usual dose is 1 inhalation twice a day.
For the treatment of COPD, the doctor may also prescribe other bronchodilators, such as anticholinergic medicines (e.g. tiotropium and ipratropium bromide).
Instructions for use
The doctor, nurse or pharmacist should demonstrate how to use the inhaler and regularly check that it is being used correctly.
The inhaler contains 60 doses of the medicine in a rolled-up foil strip. The inhaler has a dose counter that shows how many doses are left (from 60 to 0). The last 10 doses are marked on a red background.
The inhaler is not intended for multiple refilling. The used inhaler should be disposed of and replaced with a new one.
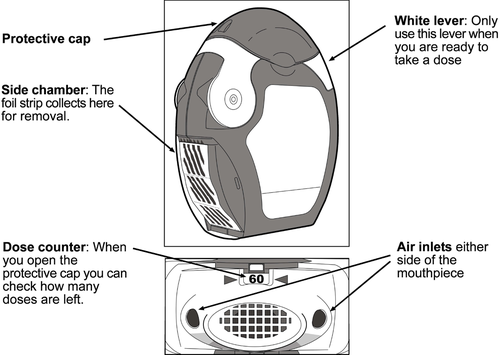
Before using the inhaler
Open the transparent side chamber cover.
Carefully tear off the entire length of the strip, using the notched edge (as shown in the picture). The strip should not be pulled or jerked.
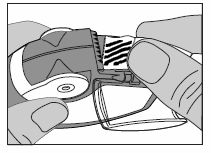
Close the side chamber cover and discard the torn strip of foil.
Important:
As the inhaler is used, the side chamber will gradually fill with used foil strips. The foil strips with black lines do not contain medicine. At the end, numbered sections of the strip will appear in the chamber.
The side chamber should not contain more than 2 strips, as this may cause the inhaler to clog. The strip of foil should be carefully torn off (as shown above) and disposed of safely.
Using the inhaler
The inhaler should be held in the hands as shown in the pictures.
1. Opening
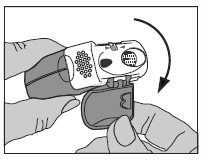
Exposing the mouthpiece by pulling down the protective cap.
Check the dose counter window to see how many doses of the medicine are left.
2. Preparing the dose
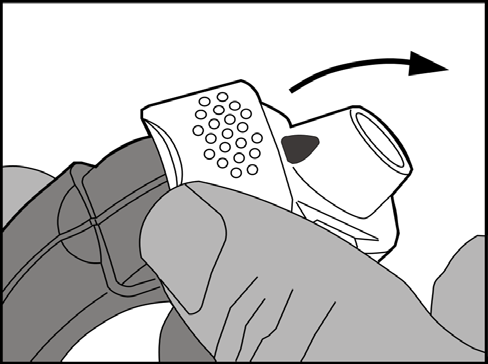
Liftthe edge of the white lever. Make sure the side chamber is closed.
Important: the white lever should only be used when the patient is ready to take a dose of the medicine. Unnecessary use of the lever will result in loss of doses.
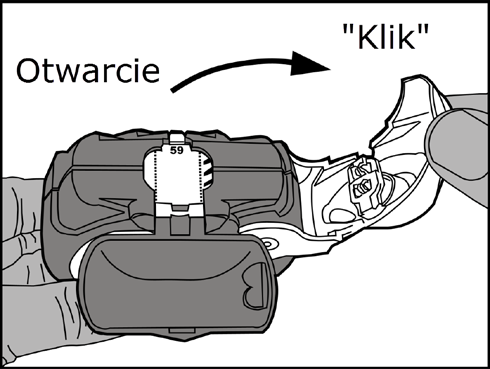
Opening: pull the white lever to the stop(until a clickis heard), which will load the dose of the medicine and display its number on the dose counter.
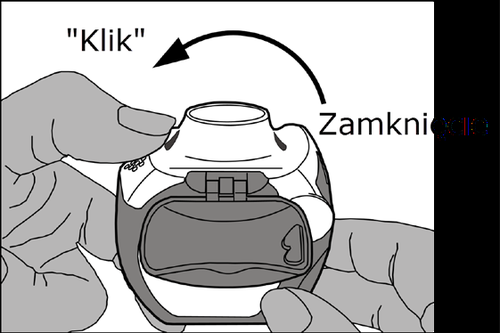
Closing:carefully closethe white lever so that a clickis heard. The inhaler is now ready for immediate use.
3. Inhaling the dose
Hold the inhaler away from the mouthpiece and exhale as deeply as possible. Never exhale into the inhaler, as this may change the size of the dose.
Hold the inhaler with the protective cap facing downwards.
Put your lips tightly around the mouthpiece.
Take a deepand forceful inhalationthrough the mouth (not the nose), as deeply as possible.
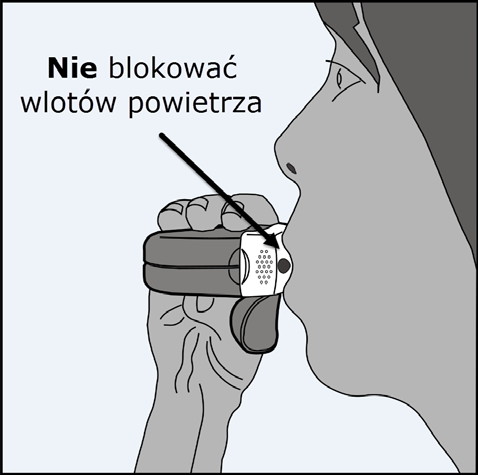
Remove the inhaler from the mouth and hold your breath for 5-10 secondsor for as long as it is comfortable.
Then breathe out slowly not in the direction of the inhaler.
Close the protective cap over the mouthpiece.
Rinse the mouth with water (spit out the water), which will help prevent the development of a fungal infection in the mouth and the occurrence of hoarseness.
Cleaning
If necessary, wipe the outer part of the mouthpiece with a clean, dry cloth.
The inhaler should not be disassembled or cleaned with water or wet cloths, as moisture may change the size of the dose!
Never insert a needle or other sharp objects into the mouthpiece or other parts of the inhaler, as this may damage it!
Using a higher than recommended dose of Airbufo Forspiro
It is important that the patient uses the medicine as directed by their doctor. They should not increase the prescribed dose without consulting their doctor.
The most common symptoms that may occur after taking a higher than recommended dose of Airbufo Forspiro are: tremors, headache or rapid heartbeat.
Missing a dose of Airbufo Forspiro
- If a dose is missed, it should be taken as soon as possible after remembering. However, if it is almost time for the next dose, the missed dose should be skipped.
- Do nottake a double dose to make up for a missed dose.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following symptoms occur, the patient should stop using Airbufo Forspiro and consult their doctor:
- Swelling of the face, especially around the mouth (swelling of the tongue and/or throat and/or difficulty swallowing) or hives with difficulty breathing (angioedema) and/or sudden feeling of fainting. These symptoms may indicate a rare allergic reaction (may occur in less than 1 in 1000 people).
- Sudden onset of wheezing or shortness of breath immediately after inhalation. If any of these symptoms occur, the patient should stop using Airbufo Forspiro and use their emergency inhaler immediately. They should consult their doctor immediately, as a change in treatment may be necessary.This situation occurs very rarely (may occur in less than 1 in 10,000 people).
Other possible side effects:
Common(may occur in less than 1 in 10 people)
palpitations (feeling of heartbeat), muscle tremors, tremors (usually mild and disappearing during continued use of Airbufo Forspiro)
thrush (fungal infection) in the mouth (the risk of its development is lower if the patient rinses their mouth with water after inhalation)
mild sore throat, cough and hoarseness
headache
pneumonia (lung infection) in patients with COPD
If any of the following symptoms occur while using Airbufo Forspiro, the patient should consult their doctor - they may be symptoms of a lung infection:
fever or chills
increased production of sputum, change in its color
worsening cough or increased breathing difficulties
Uncommon(may occur in less than 1 in 100 people)
aggression
anxiety
feeling of restlessness, nervousness or excitement
sleep disturbances
dizziness
nausea
rapid heartbeat
presence of bruises on the skin
muscle cramps
blurred vision
Rare(may occur in less than 1 in 1000 people)
rash, itching
bronchospasm (constriction of the airway muscles causing wheezing). If the patient experiences sudden wheezing after inhalation, they should stop using Airbufo Forspiro and consult their doctor immediately.
low potassium levels in the blood
irregular heartbeat
Very rare(may occur in less than 1 in 10,000 people)
depression
changes in behavior, especially in children
chest pain or tightness (angina pectoris)
increased blood sugar levels (hyperglycemia)
changes in taste, such as unpleasant taste in the mouth
changes in blood pressure
weight gain, moon face, weakness, abdominal obesity (Cushing's syndrome).
Inhaled corticosteroids, especially when used long-term in high doses, may affect the normal production of steroid hormones in the body. The symptoms include:
- changes in bone mineral density (osteoporosis)
- cataract (clouding of the lens in the eye)
- glaucoma (increased pressure in the eye)
- slowed growth rate in children and adolescents
- effect on the adrenal glands (small glands located near the kidneys)
- Cushingoid features, which include weight gain, moon face, poor wound healing, and thinning of the skin.
- increased susceptibility to infections and reduced ability to adapt to stressful situations.
The likelihood of these symptoms occurring is much lower when using inhaled corticosteroids than when taking corticosteroid tablets.
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw,
tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Airbufo Forspiro
The medicine should be stored out of sight and reach of children.
Do not store above 30°C.
Do not use this medicine after the expiry date stated on the packaging or inhaler label after EXP. The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Airbufo Forspiro contains
- The active substances are budesonide and formoterol fumarate dihydrate. Each delivered dose (inhalation dose) contains 320 micrograms of budesonide and 9 micrograms of formoterol fumarate dihydrate. Each measured dose (dose of medicine in the blister before administration) contains 346.3 micrograms of budesonide and 10.8 micrograms of formoterol fumarate dihydrate.
- The other ingredient is lactose monohydrate (contains milk proteins).
What Airbufo Forspiro looks like and contents of the pack
Airbufo Forspiro is a red and white plastic inhaler containing the medicine. Each inhaler contains a blister of OPA/Aluminium/PVC/Aluminium foil with 60 divided doses of inhalation powder.
The inhalation powder is uniform, white, off-white or light yellow.
The pack contains 1 inhaler with 60 doses.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
Manufacturer:
Aeropharm GmbH
Francois-Mitterrand-Allee 1
07407 Rudolstadt, Germany
LEK farmacevtska družba d.d.
Verovškova ulica 57
1526 Ljubljana, Slovenia
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1
39179 Barleben, Sachsen-Anhalt
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Sweden
Bofunir 320 micrograms/9 micrograms/inhalation powder, pre-dosed
Austria
AirBuFo Forspiro 320 micrograms/9 micrograms/inhalation powder, single-dose
Bulgaria
ЕрБуФо Форспиро 320 микрограма + 9 микрограма/доза прах за инхалация, предварително дозиран
AirBuFo® Forspiro® 320 micrograms + 9 micrograms/dose powder for inhalation, pre-dosed
Czech Republic
Airbufo Forspiro
Germany
Airbufo Forspiro 320 micrograms/9 micrograms/inhalation powder, single-dose
Estonia
Airbufo Forspiro
Greece
AirBuFo Forspiro, 320 μικρογραμμάρια/9 μικρογραμμάρια/εισπνοή, κόνις για εισπνοή σε δόσεις
Croatia
AirBuFo Forspiro 320/9 micrograms/dose, inhalation powder, pre-dosed
Lithuania
AirBuFo Forspiro 320 /9 micrograms/dose inhalation powder, pre-dosed
Latvia
Airbufo Forspiro 320 micrograms/9 micrograms/inhalation powder, pre-dosed
Netherlands
AirBuFo Forspiro 320/9 micrograms/dose, inhalation powder, pre-dosed
Poland
Airbufo Forspiro
Romania
Airbufo Forspiro 320 micrograms/9 micrograms/inhalation powder, single-dose
Slovenia
Airbufo Forspiro 320 micrograms/9 micrograms/dose inhalation powder, pre-dosed
Slovakia
Airbufo Forspiro 320 micrograms/9 micrograms/dose
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
tel. 22 209 70 00
Date of last revision of the leaflet:12/2021
{Logo Sandoz}
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAeropharm GmbH Lek farmacevtska družba d.d. (Lek Pharmaceuticals d.d.) Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Airbufo ForspiroDosage form: Powder, (160 mcg + 4.5 mcg)/inh. doseActive substance: formoterol and budesonidePrescription requiredDosage form: Powder, (160 mcg + 4.5 mcg)/inh. doseActive substance: formoterol and budesonideManufacturer: Orion CorporationPrescription requiredDosage form: Powder, (320 mcg + 9 mcg)/inh. doseActive substance: formoterol and budesonideManufacturer: Orion CorporationPrescription required
Alternatives to Airbufo Forspiro in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Airbufo Forspiro in Україна
Alternative to Airbufo Forspiro in Іспанія
Online doctors for Airbufo Forspiro
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Airbufo Forspiro – subject to medical assessment and local rules.