

Sudafed Xilosprai Dex

Ask a doctor about a prescription for Sudafed Xilosprai Dex

How to use Sudafed Xilosprai Dex
Package Leaflet: Information for the Patient
Sudafed Xylospray DEX, (1 mg + 50 mg)/ml, Nasal Spray, Solution
Xylometazoline Hydrochloride + Dexpanthenol
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient, or as directed by a doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 7 days (3 days in children) there is no improvement or you feel worse, consult a doctor.
Table of Contents of the Leaflet
- 1. What is Sudafed Xylospray DEX and what is it used for
- 2. Important information before using Sudafed Xylospray DEX
- 3. How to use Sudafed Xylospray DEX
- 4. Possible side effects
- 5. How to store Sudafed Xylospray DEX
- 6. Contents of the pack and other information
1. What is Sudafed Xylospray DEX and what is it used for
Sudafed Xylospray DEX nasal spray contains xylometazoline hydrochloride and dexpanthenol.
Xylometazoline hydrochloride quickly reduces the swelling of the nasal mucosa and reduces its congestion. Dexpanthenol is a derivative of vitamin, pantothenic acid, which accelerates wound healing and protects the nasal mucosa.
Sudafed Xylospray DEX is indicated:
- to reduce the swelling of the nasal mucosa in rhinitis and to support the treatment of nasal mucosa injuries,
- to alleviate the symptoms of non-allergic rhinitis (vasomotor rhinitis),
- to treat nasal congestion after nasal surgery; the use of Sudafed Xylospray DEX for this indication is possible only on the recommendation of a doctor.
Sudafed Xylospray DEX is intended for use in adults and children over 6 years of age.
2. Important information before using Sudafed Xylospray DEX
When not to use Sudafed Xylospray DEX:
- if the patient is allergic to xylometazoline hydrochloride or dexpanthenol, or any of the other ingredients of this medicine (listed in section 6),
- if the patient has dry rhinitis with crust formation (rhinitis sicca),
- in patients after surgical removal of the pituitary gland or other operations involving exposure of the meninges (membranes surrounding the brain).
Sudafed Xylospray DEX should not be used in children under 6 years of age.
Warnings and precautions
Before starting to use Sudafed Xylospray DEX, discuss it with your doctor or pharmacist:
- if the patient is taking monoamine oxidase inhibitors (MAOIs) or other medicines that may increase blood pressure,
- if the patient has increased intraocular pressure (glaucoma), especially in the case of glaucoma with a narrow angle of filtration,
- if the patient has severe cardiovascular disease (e.g. long QT syndrome, coronary heart disease, hypertension),
- if the patient has a metabolic disorder (e.g. diabetes, hyperthyroidism manifested as increased sweating, increased body temperature or accelerated heart rate),
- if the patient has a pheochromocytoma,
- if the patient has a metabolic disorder called porphyria,
- if the patient has prostatic hyperplasia.
If any of the above situations apply to the patient, before using the medicine, consult a doctor or pharmacist.
Long-term and improper use
Due to the risk of atrophy of the nasal mucosa, the medicine can be used in the period of chronic rhinitis only under the supervision of a doctor.
Improper use or administration of an excessive amount of the medicine may cause systemic side effects, especially in children (see section Possible side effects).
Long-term use or use of doses higher than recommended may lead to chronic swelling and potential atrophy (damage) of the nasal mucosa.
Avoid direct contact of the medicine with the eyes.
Children
Avoid long-term use or use of doses higher than recommended, especially in children.
Sudafed Xylospray DEX is intended for use only in children over 6 years of age. For children under 6 years of age, a nasal spray containing smaller doses of active substances is available.
The use of this medicine in children under 12 years of age should be supervised.
Sudafed Xylospray DEX and other medicines
Tell your doctor or pharmacist about all medicines you are taking now or have taken recently, as well as about medicines you plan to take.
Concomitant use of Sudafed Xylospray DEX with certain medicines for the treatment of depression (monoamine oxidase inhibitors of the tranylcypromine type or tricyclic antidepressants) and medicines that increase blood pressure may cause an increase in blood pressure due to the effect of active substances on the cardiovascular system.
Concomitant use of Sudafed Xylospray DEX with other medicines containing sympathomimetics (cough and cold medicines used to treat nasal congestion, such as pseudoephedrine, ephedrine, phenylephrine, oxymetazoline, xylometazoline, tramazoline, naphazoline, tuaminoheptane) may lead to side effects from the cardiovascular and central nervous systems.
Do not use blood pressure lowering medicines (e.g. methyldopa) with xylometazoline, which may cause an increase in blood pressure.
Consult a doctor before using Sudafed Xylospray DEX if you or your child are taking any of the above medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant or plan to have a child, consult a doctor or pharmacist before using this medicine.
Due to the lack of data on the safety of using the medicine in pregnant women, this medicine should not be used during pregnancy.
It is not known whether xylometazoline hydrochloride passes into breast milk, therefore this medicine should not be used during breastfeeding.
Driving and using machines
If the medicine is used as recommended, its use should not affect the ability to drive and use machines.
3. How to use Sudafed Xylospray DEX
This medicine should always be used exactly as described in this package leaflet for the patient, or as directed by a doctor or pharmacist. In case of doubt, consult a doctor or pharmacist.
The recommended dose of Sudafed Xylospray DEX for adults is one dose of the spray into each nostril up to three times a day, as needed, unless otherwise directed by a doctor. Do not use for more than 7 days. If after 7 days there is no improvement or you feel worse, consult a doctor. Re-use is possible only after a few days' break.
If after 3 days in a child there is no improvement or the child feels worse, consult a doctor.
Method of administration
Sudafed Xylospray DEX is intended for intranasal use.
Remove the protective cap from the tip of the dispenser.
Before first use, press the pump 5 times until a uniform mist appears. If the spray is not used for a longer period, press the pump 2 times before use.

Place the tip of the dispenser in the nostril in a position close to vertical, and then press the pump once. While dosing, gently inhale the air through the nose. If necessary, repeat the procedure in the second nostril. After each use, wipe the tip of the dispenser with a paper tissue and put on the protective cap.
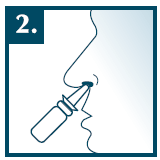
For hygiene reasons, to avoid infections, the medicine should be used by only one person.
Use in children
The recommended dose of Sudafed Xylospray DEX for children over 6 years of age is one dose of the spray into each nostril up to three times a day, as needed. To determine the duration of use in children, consult a doctor.
Use of a higher dose of Sudafed Xylospray DEX than recommended
In case of use of a higher dose of the medicine than recommended or accidental ingestion of large amounts of the medicine, the following side effects may occur:
- pupil constriction,
- pupil dilation,
- fever,
- sweating,
- pallor,
- blue discoloration of the lips (cyanosis),
- nausea,
- seizures,
- cardiovascular disorders (increased heart rate, decreased heart rate, arrhythmias, heart failure, cardiac arrest, hypertension),
- respiratory disorders (pulmonary edema, respiratory disorders),
- psychiatric disorders,
- drowsiness, decreased body temperature, slowed heart rate, and decreased blood pressure, respiratory arrest, and coma.
If any of these symptoms occur, consult a doctor immediately.
Missing a dose of Sudafed Xylospray DEX
Do not use a double dose to make up for a missed dose. Continue using as described in the leaflet.
In case of any further doubts related to the use of this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Uncommon (may affect up to 1 in 100 people):
- hypersensitivity reactions, such as skin and mucous membrane swelling, rash, itching.
Rare (may affect up to 1 in 1,000 people):
- palpitations (feeling of heartbeat), tachycardia (accelerated heart rate), hypertension (high blood pressure).
Very rare (may affect up to 1 in 10,000 people):
- restlessness, difficulty falling asleep, fatigue (drowsiness, sedation, headache, hallucinations (mainly in children)),
- arrhythmias,
- nasal mucosa swelling (after stopping treatment), nosebleeds,
- seizures (mainly in children).
Frequency not known (frequency cannot be estimated from the available data):
- burning and dryness of the nasal mucosa, sneezing.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warszawa, tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Sudafed Xylospray DEX
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after: EXP.
The expiry date refers to the last day of the month stated.
Do not store above 25°C.
Shelf life after first opening of the bottle: 6 months.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Sudafed Xylospray DEX contains
- The active substances of the medicine are xylometazoline hydrochloride and dexpanthenol. Each ml of the solution contains 1 mg of xylometazoline hydrochloride and 50 mg of dexpanthenol. One dose of the nasal spray (0.1 ml of the solution) contains 0.1 mg of xylometazoline hydrochloride and 5.0 mg of dexpanthenol.
- The other ingredients are: potassium dihydrogen phosphate, disodium phosphate dodecahydrate, water for injections.
What Sudafed Xylospray DEX looks like and contents of the pack
Clear, colorless to slightly yellowish solution, free from particles.
The packaging of Sudafed Xylospray DEX is a 10 ml HDPE bottle with a dosing pump in a cardboard box.
10 ml of the solution is sufficient for 80 doses.
Marketing authorization holder and manufacturer
Marketing authorization holder:
McNeil Healthcare (Ireland) Limited
Office 5, 6 & 7, Block 5
High Street, Tallaght
Dublin 24, D24 YK8N
Ireland
Manufacturer:
Famar Health Care Services Madrid S.A.U.
Avda Leganés, 62, Alcorcón
28923 Madrid
Spain
Ursapharm Arzneimittel GmbH
Industriestraße 35
66129 Saarbrücken
Germany
Johnson & Johnson GmbH
Johnson & Johnson Platz 2
41470 Neuss
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Latvia
Olydex
Poland
Sudafed Xylospray DEX
Slovakia
Olynth Plus
Lithuania
Olydex
Germany
Olynth Plus 0,1% / 5%
Estonia
Sudafed Xylospray DEX
Czech Republic
Olynth Plus
Portugal
Nasex Duo
Italy
ACTIFED DECONGESTIONANTE LENITIVO
Romania
Olynth Duo
Bulgaria
Olynth Duo
Slovenia
Olynth Combo
Croatia
Olynth Combo
Greece
HEXARHINAL PLUS
Cyprus
HEXARHINAL PLUS
Hungary
Actifed
To obtain more detailed information, please contact:
email: [email protected]
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterFamar Health Care Services Madrid SAU Johnson & Johnson GmbH Ursapharm Arzneimittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Sudafed Xilosprai DexDosage form: Aerosol, (0.05 mg + 5 mg)/doseActive substance: xylometazolineManufacturer: Klosterfrau Berlin GmbHPrescription not requiredDosage form: Aerosol, (0.1 mg + 5 mg)/doseActive substance: xylometazolineManufacturer: Klosterfrau Berlin GmbHPrescription not requiredDosage form: Aerosol, (0.5 mg + 0.6 mg)/mlActive substance: xylometazolinePrescription not required
Alternatives to Sudafed Xilosprai Dex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Sudafed Xilosprai Dex in Ukraine
Alternative to Sudafed Xilosprai Dex in Spain
Online doctors for Sudafed Xilosprai Dex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Sudafed Xilosprai Dex – subject to medical assessment and local rules.








