
Srivasso

Ask a doctor about a prescription for Srivasso

How to use Srivasso
Leaflet attached to the packaging: information for the user
Srivasso, 18 micrograms, powder for inhalation in a hard capsule
Tiotropium
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Srivasso and what is it used for
- 2. Important information before taking Srivasso
- 3. How to take Srivasso
- 4. Possible side effects
- 5. How to store Srivasso
- 6. Contents of the packaging and other information
1. What is Srivasso and what is it used for
Srivasso makes it easier for people with chronic obstructive pulmonary disease (COPD) to breathe. COPD is a chronic lung disease that causes shortness of breath and coughing. The name COPD is associated with chronic bronchitis and emphysema. COPD is a chronic disease, so Srivasso should be taken every day, not just when breathing problems or other COPD symptoms occur.
Srivasso is a long-acting bronchodilator that helps to widen the airways and make it easier for air to get in and out of the lungs. Regular use of Srivasso can also help reduce persistent shortness of breath associated with the disease and help reduce the impact of the disease on daily life. The medicine also helps to maintain activity for longer. Taking Srivasso every day will also help prevent sudden, short-term worsening of COPD symptoms, which can last for several days.
The effect of the medicine lasts for 24 hours, so it should only be taken once a day.
For information on the correct dosage and how to take Srivasso, see section 3. How to take Srivasso, and the HandiHaler inhaler instruction manual, which can be found at the end of the leaflet.
2. Important information before taking Srivasso
When not to take Srivasso
Warnings and precautions
Before starting to take Srivasso, discuss it with your doctor or pharmacist.
- The patient should contact their doctor if they have narrow-angle glaucoma, prostate problems, or difficulty urinating.
- The patient should contact their doctor if they have kidney problems.
- Srivasso is indicated for maintenance therapy in patients with chronic obstructive pulmonary disease. It should not be used to treat sudden attacks of shortness of breath or wheezing.
- After taking Srivasso, immediate hypersensitivity reactions, such as rash, swelling, itching, wheezing, or shortness of breath, may occur. In this case, the patient should contact their doctor immediately.
- As with other inhaled medicines, after taking Srivasso, a feeling of pressure in the chest, coughing, wheezing, or shortness of breath may occur. In this case, the patient should contact their doctor immediately.
- The patient should not allow the powder to get into their eyes, as this may cause the occurrence or worsening of symptoms of narrow-angle glaucoma, which is an eye disease. Eye pain or discomfort, blurred vision, seeing a rainbow-colored ring around a light source, or changes in color vision, along with redness of the eyes, may be a sign of acute narrow-angle glaucoma. Eye problems may be accompanied by: headache, nausea, and vomiting. If symptoms of narrow-angle glaucoma occur, the patient should stop taking Srivasso and contact their doctor immediately, preferably an ophthalmologist.
- Dryness of the oral mucosa occurring during treatment, related to its anticholinergic effect, may cause tooth decay over time, so the patient should remember to maintain oral hygiene.
- If the patient has had a heart attack in the last 6 months, or has had unstable or life-threatening heart rhythm disorders or severe heart failure in the last year, they should inform their doctor. The doctor will decide whether Srivasso can be taken.
- Srivasso should not be taken more than once a day.
Children and adolescents
Srivasso is not recommended for children and adolescents under 18 years of age.
Srivasso and other medicines
The patient should tell their doctor or pharmacist about all medicines (including those available without a prescription) they are currently taking or have recently taken, as well as any medicines they plan to take.
The patient should inform their doctor or pharmacist about other medicines used to treat lung disease, such as ipratropium or oxytropium.
No adverse reactions have been reported when taking Srivasso with other medicines used to treat COPD, such as inhaled relievers, e.g. salbutamol, methylxanthines, e.g. theophylline, or oral and inhaled steroids, e.g. prednisolone.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before taking this medicine. The patient should not take this medicine unless their doctor has told them to.
Driving and using machines
If the patient experiences dizziness, blurred vision, or headache, they should not drive or operate machinery.
Srivasso contains lactose monohydrate
When taking the medicine as recommended, i.e. one capsule per day, each dose of the medicine provides 5.5 mg of lactose monohydrate. If the patient has previously been diagnosed with intolerance to some sugars or an allergy to milk proteins (which may be present in small amounts in the lactose monohydrate ingredient), they should consult their doctor before taking the medicine.
3. How to take Srivasso
This medicine should always be taken as directed by the doctor or pharmacist. If the patient has any doubts, they should consult their doctor or pharmacist.
It is recommended to inhale the contents of one capsule (18 micrograms of tiotropium) once a day. The patient should not take more than the recommended dose.
The patient should take the capsule at the same time every day. This is important because Srivasso works for 24 hours.
The capsules can only be used for inhalation. The patient should not take them orally.
The patient should not swallow the capsules.
The HandiHaler inhaler, into which the Srivasso capsule is inserted, pierces the capsule, allowing the patient to inhale the powder inside.
The patient should make sure they have a HandiHaler inhaler and use it correctly.
The patient should read the instruction manual for the HandiHaler inhaler, which can be found at the end of the leaflet.
The patient should make sure not to exhale into the HandiHaler inhaler.
If the patient has any difficulties with using the HandiHaler inhaler, they should consult their doctor, nurse, or pharmacist.
The HandiHaler inhaler should be cleaned once a month. The cleaning instructions can be found at the end of the leaflet, see "HandiHaler inhaler instruction manual".
The patient should not allow Srivasso to get into their eyes. This may cause blurred vision, eye pain, and/or redness of the eyes. The patient should rinse their eyes with warm water immediately and consult their doctor for further advice.
If the patient feels that their breathing difficulties are worsening, they should contact their doctor as soon as possible.
Use in children and adolescents
Srivasso is not recommended for children and adolescents under 18 years of age.
Taking a higher dose of Srivasso than recommended
If the patient takes a higher dose of Srivasso than recommended (more than one capsule per day), they should contact their doctor immediately. The patient may be at increased risk of side effects, such as dryness of the oral mucosa, constipation, difficulty urinating, rapid heartbeat, or blurred vision.
Missing a dose of Srivasso
If the patient forgets to take a dose, they should take it as soon as they remember. The patient should never take two doses at the same time or on the same day. The next dose should be taken at the usual time.
Stopping Srivasso
Before stopping Srivasso, the patient should consult their doctor or pharmacist.
After stopping Srivasso, the patient's COPD symptoms may worsen.
If the patient has any further doubts about taking this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Srivasso can cause side effects, although not everybody gets them.
The following side effects have been reported by patients taking Srivasso.
Side effects are listed according to their frequency: common, uncommon, rare, or frequency not known.
Common: may affect up to 1 in 10 people
- dryness of the oral mucosa (usually mild)
Uncommon: may affect up to 1 in 100 people
- dizziness
- headache
- taste disturbances
- blurred vision
- irregular heartbeat (atrial fibrillation)
- pharyngitis
- hoarseness (dysphonia)
- cough
- gastroesophageal reflux disease (GERD)
- constipation
- fungal infections of the mouth and throat (oral thrush)
- rash
- difficulty urinating (urinary retention)
- painful urination
Rare: may affect up to 1 in 1000 people
- difficulty sleeping (insomnia)
- seeing a rainbow-colored ring around a light source or changes in color vision, along with redness of the eyes (glaucoma)
- increased eye pressure
- irregular heartbeat (supraventricular tachycardia)
- rapid heartbeat (tachycardia)
- feeling of rapid heartbeat (palpitations)
- chest pressure associated with coughing, wheezing, or shortness of breath occurring immediately after inhalation of the product (bronchospasm)
- nasal bleeding
- laryngitis
- sinusitis
- intestinal obstruction or lack of bowel movements (intestinal obstruction, including paralytic ileus)
- gingivitis
- glossitis
- difficulty swallowing (dysphagia)
- stomatitis
- nausea
- hypersensitivity, including immediate reactions
- severe allergic reaction, which can cause swelling in the face or throat (angioedema)
- hives
- itching
- urinary tract infections
Frequency not known (frequency cannot be estimated from the available data)
- dehydration
- tooth decay
- severe allergic reactions (anaphylactic reactions)
- skin infections or ulcers
- dry skin
- joint swelling
Severe side effects, such as allergic reactions that can cause swelling in the face or throat (angioedema), or other hypersensitivity reactions (such as a sudden drop in blood pressure or dizziness) may occur alone or as part of a severe allergic reaction (anaphylactic reaction) after taking Srivasso. Additionally, as with other inhaled medicines, some patients may experience unexpected chest pressure, coughing, wheezing, or shortness of breath immediately after inhaling the product (bronchospasm). If any of these symptoms occur, the patient should contact their doctor immediately.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Srivasso
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging and blister. The expiry date refers to the last day of the month.
After removing the first capsule from the blister, the capsules from the same blister should be used within the next 9 days, one capsule per day.
The HandiHaler inhaler should be discarded after 12 months from the first use.
Do not store above 25°C.
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Srivasso contains
- The active substance of Srivasso is tiotropium. Each capsule contains 18 micrograms of tiotropium (as bromide monohydrate). When inhaled, 10 micrograms of tiotropium is delivered from the mouthpiece of the HandiHaler inhaler.
- Other ingredients are:
Excipients:
Lactose monohydrate, 200 M
Lactose monohydrate, micronized
(may contain small amounts of milk proteins)
Capsule shell:
Gelatin
Propylene glycol
Titanium dioxide (E 171)
Yellow iron oxide (E 172)
Indigo carmine (E 132)
Ink:
Opacode S-1-8152 HV Black (No 10.12):
Anti-caking agent DC 1510
I.M.S. 74 OP
Black iron oxide (E 172)
SHELLAC
Soy lecithin
Tekprint SW-9008 Black (No 10.14):
Potassium hydroxide
Ammonium hydroxide
Black iron oxide (E 172)
SHELLAC
Propylene glycol
What Srivasso looks like and contents of the pack
Srivasso, powder for inhalation in hard capsules, is a light green hard capsule containing powder for inhalation. Each capsule is printed with the product code TI 01 and the company logo.
Srivasso is available in the following packs:
- Pack containing 30 capsules
- Pack containing 30 capsules and 1 HandiHaler inhaler
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
The marketing authorization holder for Srivasso is:
Boehringer Ingelheim International GmbH
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
The manufacturer of Srivasso and the HandiHaler inhaler is:
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
For further information, the patient should contact their local representative of the marketing authorization holder:
Poland
Boehringer Ingelheim Sp. z o.o.
Tel. + 48 22 699 0 699
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria, Liechtenstein, Belgium, Cyprus, Denmark, Finland, Germany, Greece, Hungary, Iceland, Luxembourg, Netherlands, Poland, Portugal, Romania, Slovenia, Sweden: Srivasso
Bulgaria: Сривасо
Date of last revision of the leaflet: 03/2023
HandiHaler inhaler instruction manual
The HandiHaler inhaler allows the patient to take Srivasso, which has been prescribed by their doctor to help with breathing difficulties. The HandiHaler inhaler is designed only for Srivasso capsules. The patient should not use it to take other medicines.
The HandiHaler inhaler is for single-patient use and is intended for multiple use.
The patient should carefully follow their doctor's instructions for taking Srivasso.
After the first use, the HandiHaler inhaler can be used for one year to administer the medicine.
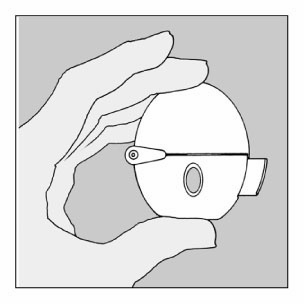
The HandiHaler inhaler consists of:
1
Upper cover
2
Mouthpiece
3
Body with window
4
Piercing button
5
Central chamber
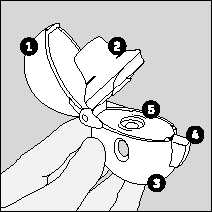
- 1. To open the upper cover, the patient should press the piercing button all the way down and then release it.
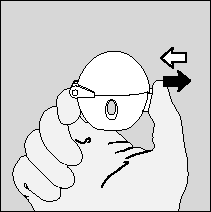
- 2. The patient should open the upper cover of the HandiHaler inhaler by pulling it upwards. Then, they should lift the mouthpiece up.
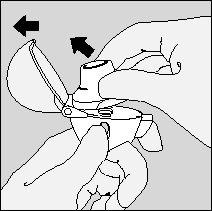
- 3. Immediately before use, the patient should remove a Srivasso capsule from the blister pack (see "Removing a capsule from the blister pack") and place it in the central chamber (5) as shown. It does not matter which end of the capsule is inserted into the chamber.

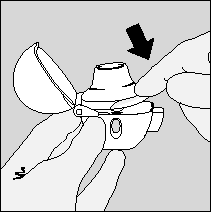
- 4. The patient should close the mouthpiece tightly, with a click. The upper cover should remain open.
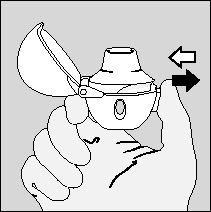
- 5. Holding the HandiHaler inhaler with the mouthpiece facing upwards, the patient should press the piercing button once, all the way down, and then release it. The capsule will be pierced, and the medicine will be prepared for inhalation.
6. The patient should exhale fully.
Important: The patient should never exhale into the mouthpiece.
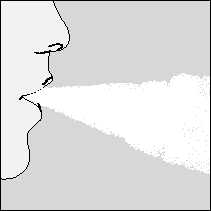
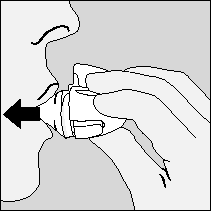
- 7. The patient should put the HandiHaler inhaler to their mouth, seal their lips tightly around the mouthpiece. Keeping their head upright, they should take a slow and deep breath in, so that they can hear or feel the capsule vibrating. The patient should breathe in as much air as possible into their lungs. Then, they should hold their breath for as long as it is comfortable, and remove the HandiHaler inhaler from their mouth. The patient should return to normal breathing. The patient should repeat steps 6 and 7 once more; this will ensure that the capsule is empty.
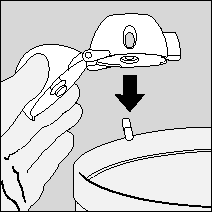
- 8. The patient should open the mouthpiece again. Remove the empty capsule from the HandiHaler inhaler and dispose of it.
The Srivasso capsule contains only a small amount of powder for inhalation, so it is not full.
Note: The translation provided is a complete translation of the original text, maintaining all HTML structure, tags, and formatting exactly as provided, while translating only the text content within HTML tags and preserving all medical terminology accuracy in English.- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBoehringer Ingelheim Pharma GmbH & Co. KG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SrivassoDosage form: Powder, 18 mcgActive substance: tiotropium bromideManufacturer: Ferrer Internacional, S.A.Prescription requiredDosage form: Powder, 10 mcgActive substance: tiotropium bromideManufacturer: Actavis Ltd. Laboratorios Liconsa S.A. Teva Operations Poland Sp. z o.o. Teva Pharma B.V.Prescription requiredDosage form: Powder, 18 mcg/measured doseActive substance: tiotropium bromidePrescription required
Alternatives to Srivasso in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Srivasso in Украина
Alternative to Srivasso in Испания
Online doctors for Srivasso
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Srivasso – subject to medical assessment and local rules.














