
Smofkabiven Ef

Ask a doctor about a prescription for Smofkabiven Ef

How to use Smofkabiven Ef
Leaflet attached to the packaging: information for the user
SmofKabiven EF, infusion emulsion
Read the leaflet carefully before using the medicine, as it contains
important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What SmofKabiven EF is and what it is used for
- 2. Important information before using SmofKabiven EF
- 3. How to use SmofKabiven EF
- 4. Possible side effects
- 5. How to store SmofKabiven EF
- 6. Contents of the packaging and other information
1. What SmofKabiven EF is and what it is used for
SmofKabiven EF is an infusion emulsion administered to the patient through a drip (intravenous infusion). The packaging of the medicine consists of a plastic bag containing amino acids (essential components for protein production), glucose (carbohydrates), and fats (lipids). The medicine can be used in adult patients and children over 2 years of age.
Medical professionals administer SmofKabiven EF when other methods of nutrition are insufficient or impossible.
2. Important information before using SmofKabiven EF
Do not use SmofKabiven EF if the patient has:
- allergy to active substances or any of the other ingredients of this medicine (listed in section 6);
- allergy to fish protein or eggs;
- allergy to peanuts or soy (SmofKabiven EF contains soybean oil);
- too high a level of fats in the blood (hyperlipidemia);
- severe liver function disorders;
- blood coagulation problems (coagulation disorders);
- amino acid metabolism disorder;
- severe kidney disease, without the possibility of dialysis;
- acute shock;
- uncontrolled, increased blood sugar levels (hyperglycemia);
- fluid in the lungs (acute pulmonary edema);
- too much fluid in the body (overhydration);
- untreated heart failure;
- blood coagulation disorder (hemophagocytic syndrome);
- unstable general condition, e.g. severe post-traumatic condition, uncontrolled diabetes, acute myocardial infarction, stroke, thrombosis, metabolic acidosis (a condition characterized by too much acidic substance in the blood), severe infection (severe sepsis), coma, fluid deficiency (hypotonic dehydration).
SmofKabiven EF should not be used in children under 2 years of age.
Warnings and precautions
Before starting SmofKabiven EF, discuss with your doctor if the patient has:
- kidney disease;
- diabetes;
- pancreatitis;
- liver disease;
- thyroid dysfunction (thyroid disorders);
- sepsis (severe infection).
If during infusion fever, rash, swelling, breathing difficulties, chills, sweating, nausea, or vomiting occur, immediately inform the medical staff, as these symptoms may be caused by an allergic reaction or too high a dose of the medicine.
The doctor may recommend regular blood tests to determine liver function tests and other values.
Children and adolescents
SmofKabiven EF is not intended for administration to newborns or children under 2 years of age.
SmofKabiven EF can be administered to children from 2 to 16/18 years of age.
SmofKabiven EF and other medicines
Tell your doctor about all medicines the patient is currently taking or has recently taken, as well as medicines the patient plans to take, including those available without a prescription.
Pregnancy and breastfeeding
There is no data on the use of SmofKabiven EF during pregnancy or breastfeeding.
SmofKabiven EF is administered to pregnant or breastfeeding women only if the doctor considers it necessary. SmofKabiven EF during pregnancy and breastfeeding may be administered on the doctor's instructions.
Driving and using machines
Not applicable, as SmofKabiven EF is used in a hospital.
3. How to use SmofKabiven EF
This medicine should always be used as directed by the doctor. In case of doubts, consult a doctor.
The doctor selects an individual dose based on the patient's body weight and clinical condition. SmofKabiven EF is administered only by medical professionals.
Using a higher dose of SmofKabiven EF than recommended
It is unlikely that the patient will receive too high a dose of SmofKabiven EF, as the medicine is administered by medical professionals.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects(may occur in up to 1 in 10 patients):
slight increase in body temperature.
Uncommon side effects(may occur in up to 1 in 100 patients): high levels of liver enzymes in the blood, loss of appetite, nausea, vomiting, chills, dizziness, and headaches.
Rare side effects(may occur in up to 1 in 1000 patients): low or high blood pressure, breathing difficulties, rapid heart rate (tachycardia). Allergic reactions (which can cause symptoms such as swelling, fever, drop in blood pressure, skin rash, blisters (raised, red areas), redness, headache). Feeling hot and cold. Paleness. Slight cyanosis of the lips and skin (related to hypoxia). Neck, back, bone, chest, and lumbar pain.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181C, 02-222 Warsaw tel.: +48 22 49 21 301, fax: +48 22 49 21 309 website: https://smz.ezdrowie.gov.pl Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store SmofKabiven EF
Store the medicine out of sight and reach of children.
Do not store above 25°C. Store in the outer bag. Do not freeze.
Do not use this medicine after the expiry date stated on the bag and carton. The expiry date refers to the last day of the month stated.
6. Contents of the packaging and other information
What SmofKabiven EF contains
The active substances of the medicine are:
per 1000 ml
alanine
7.1
arginine
6.1
glycine
5.6
histidine
1.5
isoleucine
2.5
leucine
3.8
lysine (as acetate)
3.4
methionine
2.2
phenylalanine
2.6
proline
5.7
serine
3.3
taurine
0.5
threonine
2.2
tryptophan
1.0
tyrosine
0.20
valine
3.1
glucose (in the form of a monohydrate)
127
purified soybean oil
11.4
triglycerides of saturated fatty acids with medium chain length
11.4
purified olive oil
9.5
fish oil rich in omega-3 fatty acids
5.7
The other ingredients (excipients) are: glycerol, purified egg phospholipids, all-rac-α-tocopherol, sodium hydroxide (to adjust pH), sodium oleate, glacial acetic acid (to adjust pH), hydrochloric acid (to adjust pH), and water for injections.
What SmofKabiven EF looks like and what the pack contains
The glucose and amino acid solutions are clear, colorless to slightly yellow, and free of particles. The fat emulsion is white and homogeneous.
Pack sizes:
1 × 493 ml, 6 × 493 ml
1 × 986 ml, 4 × 986 ml
1 × 1477 ml, 4 × 1477 ml
1 × 1970 ml, 4 × 1970 ml
1 × 2463 ml, 3 × 2463 ml
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Fresenius Kabi AB
SE-751 74 Uppsala
Sweden
Manufacturer
Fresenius Kabi Austria GmbH
Hafnerstrasse 36
8055 Graz
Austria
Fresenius Kabi AB
SE-751 74 Uppsala
Sweden
To obtain more detailed information, please contact the representative of the marketing authorization holder:
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
tel.: +48 22 345 67 89
Date of last revision of the leaflet:27.01.2023
---------------------------------------------------------------------------------------------------------------------------
Information intended exclusively for healthcare professionals:
Warnings and precautions for use
In order to avoid hazards associated with infusion at a rate greater than recommended, it is recommended to perform it continuously and under appropriate control, if possible using a volumetric pump.
Since the use of a central vein for infusion is associated with an increased risk of infection, when inserting and handling the catheter, it is recommended to strictly follow the principles of aseptic procedure to avoid any infection.
It is recommended to monitor glucose and electrolyte levels in serum, osmolality, and fluid and acid-base balance, as well as perform liver enzyme tests.
In the event of any signs or symptoms of anaphylactic reaction (such as fever, chills, rash, or shortness of breath), the infusion should be stopped immediately.
SmofKabiven EF should not be administered simultaneously with blood in the same infusion set, due to the risk of pseudoagglutination.
Method of administration
Intravenous administration, infusion into a central vein.
In order to ensure complete parenteral nutrition, it is recommended to add microelements, electrolytes, and vitamins to SmofKabiven EF, according to the patient's needs.
Dosage
Adult patients
Recommended dosage
The dose range is from 13 to 31 ml of SmofKabiven EF/kg body weight/day, which provides from 0.6 to 1.6 g of amino acids/kg body weight/day (which corresponds to from 0.10 to 0.25 g of nitrogen/kg body weight/day) and from 14 to 35 kcal/kg body weight/day of total energy (from 12 to 27 kcal/kg body weight/day of non-protein energy).
Infusion rate
The maximum infusion rate of glucose is 0.25 g/kg body weight/hour, amino acids 0.1 g/kg body weight/hour, and fats 0.15 g/kg body weight/hour.
The infusion rate should not exceed 2.0 ml/kg body weight/hour (which corresponds to 0.25 g of glucose, 0.10 g of amino acids, and 0.08 g of fats/kg body weight/hour). The recommended infusion time is from 14 to 24 hours.
Maximum daily dose
The maximum daily dose depends on the patient's clinical condition and may change even from day to day.
The recommended maximum daily dose is 35 ml/kg body weight/day.
Children and adolescents
Children aged 2-11 years
Recommended dosage
A dose of up to 35 ml/kg body weight/day should be regularly adjusted to the requirements of the pediatric patient, which may vary significantly more than in adult patients.
Infusion rate
The recommended maximum infusion rate is 2.4 ml/kg body weight/hour (which corresponds to 0.12 g of amino acids/kg body weight/hour, 0.30 g of glucose/kg body weight/hour, and 0.09 g of fats/kg body weight/hour).
Except in special situations requiring careful monitoring, when using the recommended maximum infusion rate, the infusion time should not exceed 14 hours and 30 minutes.
The recommended infusion time is from 12 to 24 hours.
Maximum daily dose
The maximum daily dose is variable depending on the patient's clinical condition and may change even from day to day. The recommended maximum daily dose is 35 ml/kg body weight/day.
Adolescents aged 12-16/18 years
In adolescents, SmofKabiven EF can be dosed as in adult patients.
Special precautions for disposal and preparation of the medicine for use
Do not use if the packaging is damaged.
Use only if the amino acid and glucose solutions are clear, colorless to slightly yellow, and the fat emulsion is white and homogeneous. The contents of the three separate chambers should be mixed before use, as well as before adding other substances through the dedicated port.
After removing the protective covers, roll the bag several times to mix all the components of the medicine and obtain a homogeneous mixture, in which no signs of phase separation are visible. The medicine is for single use only. Any unused medicine remaining after infusion should be destroyed.
Compatibility
Compatibility data are available for Dipeptiven, Supliven/Addamel N, Glycophos, Addiphos, Vitalipid N Adult/Infant, and Soluvit N in specified quantities and electrolyte concentrations.
When adding electrolytes, consider the amounts already present in the bag to meet the patient's clinical needs. Available data confirm the possibility of adding the above-mentioned medicines to the activated bag in accordance with the table below:
Compatibility range: stable for 8 days, i.e. 6 days stored at a temperature of 2-8°C, and then 48 hours at a temperature of 20-25°C.
| Unit | Maximum total content | |||||
| SmofKabiven EF bag size | ml | 493 | 986 | 1477 | 1970 | 2463 |
| Addition | Volume | |||||
| Dipeptiven | ml | 0-100 |
|
|
|
|
| Supliven/Addamel N | ml |
|
|
|
|
|
| Soluvit N | ampoule |
|
|
|
|
|
| Vitalipid N Adult/Infant | ml |
|
|
|
|
|
| Electrolyte limits | Amount per bag | |||||
| Sodium | mmol | ≤ 75 | ≤ 150 | ≤ 225 | ≤ 300 | ≤ 375 |
| Potassium | mmol | ≤ 75 | ≤ 150 | ≤ 225 | ≤ 300 | ≤ 375 |
| Calcium | mmol | ≤ 2.5 | ≤ 5 | ≤ 7.5 | ≤ 10 | ≤ 12.5 |
| Magnesium | mmol | ≤ 2.5 | ≤ 5 | ≤ 7.5 | ≤ 10 | ≤ 12.5 |
| Inorganic phosphate (Addiphos) or organic phosphate (Glycophos) | mmol | ≤ 7.5 | ≤ 15 | ≤ 22.5 | ≤ 30 | ≤ 37.5 |
| Zinc | mmol | ≤ 0.1 | ≤ 0.2 | ≤ 0.25 | ≤ 0.3 | ≤ 0.35 |
| Selenium | μmol | ≤ 1 | ≤ 1 | ≤ 1 | ≤ 1 | ≤ 1.15 |
Note: This table is intended to demonstrate compatibility. It does not provide dosing guidelines.
Before prescribing the above-mentioned medicines, read the approved package leaflets.
Information on compatibility with other additives and storage periods of different mixtures will be available on request.
Any additives should be combined with the medicine under aseptic conditions.
Shelf life after mixing the contents of the bag
Physical and chemical stability of the mixed contents of the triple-chamber bag has been demonstrated for 48 hours at a temperature of 20-25°C. From a microbiological point of view, the medicine should be used immediately.
Otherwise, the user is responsible for the storage period during use and the storage conditions before use. This period should not normally exceed 24 hours at a temperature of 2-8°C, unless mixing took place in controlled and validated aseptic conditions.
Shelf life after mixing with additional substances
Physical and chemical stability of the mixed contents of the triple-chamber bag with additional substances has been demonstrated for up to 8 days, i.e. 6 days at a temperature of 2-8°C, and then 48 hours at a temperature of 20-25°C, including the infusion time. From a microbiological point of view, the medicine should be used immediately after adding other components. Otherwise, the user is responsible for the storage period during use and the storage conditions before use. This period should not normally exceed 24 hours at a temperature of 2-8°C, unless mixing took place in controlled and validated aseptic conditions.
SmofKabiven EF - Instructions for preparing the bag for use
Bag
493 ml
986 ml, 1477 ml, 1970 ml, 2463 ml
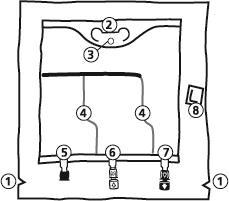
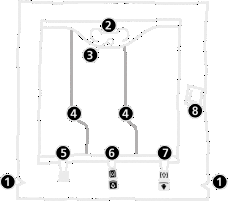
- 1. Notch in the outer bag
- 2. Bag handle
- 3. Hole for hanging the bag
- 4. Welds separating the individual chambers of the bag
- 5. Blind port (used only in production)
- 6. Port for administering additional substances
- 7. Infusion port
- 8. Oxygen absorber
| |
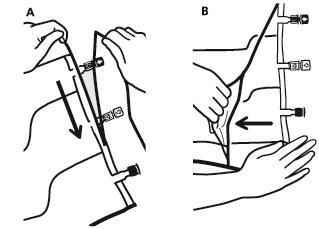 | |
- To remove the outer bag, place it horizontally and tear it along the notch (A) and the top edge.
- Then tear the outer bag along the long edge, remove it, and discard it along with the oxygen absorber (B).
2. Mixing
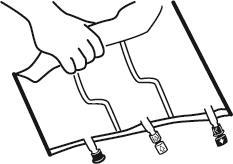
- Place the bag on a flat surface.
- Starting from the handle side, roll the bag firmly towards the ports, first with the right hand and then with the left hand, applying constant pressure, until the vertical welds rupture. The welds can also be opened before removing the outer bag. Note:the liquid mixes easily, even though the horizontal weld remains intact.
493 ml, 986 ml, 1477 ml, 1970 ml, 2463 ml
- Mix the contents of the three chambers by turning the bag three times, which should ensure thorough mixing of the components.
| |
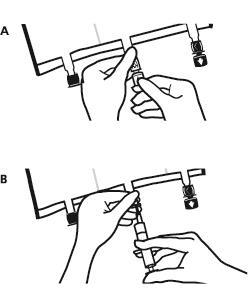 | |
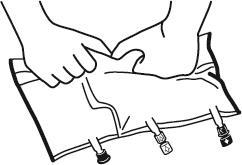
- Place the bag on a flat, even surface. Just before administering additional substances, remove the labeled plug from the white port for administering additional substances (A). Note:the membrane of the port for administering additional substances is sterile.
- Hold the base of the port for administering additional substances. Introduce the needle and inject the additional substances (with known compatibility) through the center of the injection site (B).
- Mix the contents of the bag thoroughly after adding each component by turning the bag three times after each addition. Use syringes with needles with a diameter of 18 to 23 G and a maximum length of 40 mm.
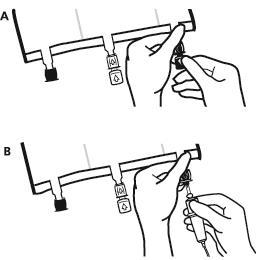
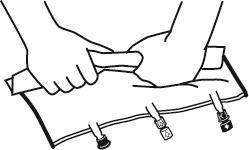
- Just before connecting the infusion set, remove the plug from the blue infusion port (A). Note:the membrane of the infusion port is sterile.
- Use infusion sets without an air vent or close the air vent.
- Hold the base of the infusion port.
- Insert the spike of the infusion set into the infusion port. To ensure good fixation of the spike, insert its entire length. Note:the inner surface of the infusion port is sterile.
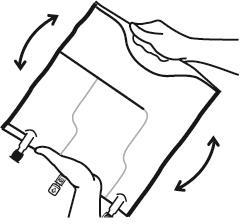
4. Hanging the bag
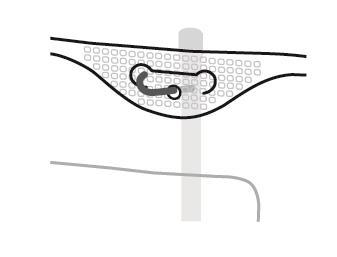
- Hang the bag using the hole located below the handle.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterFresenius Kabi AB Fresenius Kabi Austria GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Smofkabiven EfDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not required
Alternatives to Smofkabiven Ef in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Smofkabiven Ef in Spain
Alternative to Smofkabiven Ef in Ukraine
Online doctors for Smofkabiven Ef
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Smofkabiven Ef – subject to medical assessment and local rules.














