
Ropivacaine Bioq

Ask a doctor about a prescription for Ropivacaine Bioq

How to use Ropivacaine Bioq
Leaflet accompanying the packaging: patient information
Ropivacaine BioQ, 2 mg/ml, solution for infusion in an administration set
ropivacaine hydrochloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Ropivacaine BioQ and what is it used for
- 2. Important information before using Ropivacaine BioQ
- 3. How to use Ropivacaine BioQ
- 4. Possible side effects
- 5. How to store Ropivacaine BioQ
- 6. Contents of the packaging and other information
1. What is Ropivacaine BioQ and what is it used for
The name of the medicine is Ropivacaine BioQ, 2 mg/ml, solution for infusion in an administration set. It contains the active substance ropivacaine hydrochloride, which belongs to a group of medicines called local anesthetics.
Ropivacaine BioQ is used in adults for the treatment of acute pain. It causes numbness of different parts of the body, e.g., after surgery.
2. Important information before using Ropivacaine BioQ
When not to use Ropivacaine BioQ
- by injection into a blood vessel, spine, or joint to anesthetize a specific area of the body or into the cervix to relieve pain during childbirth.
If the patient is unsure whether the above warnings apply to them, they should consult their doctor before using Ropivacaine BioQ.
Warnings and precautions
Before starting treatment with Ropivacaine BioQ, the patient should discuss it with their doctor or nurse, especially if:
- the patient has heart, liver, or kidney disease;
- the patient or a family member has porphyria, a rare blood pigment disorder; the doctor should be informed, as a different medicine may be necessary;
- the patient has other diseases or conditions.
Ropivacaine BioQ and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. Ropivacaine BioQ may affect the action of some other medicines, and some medicines may affect the action of Ropivacaine BioQ.
In particular, the patient should inform their doctor if they are taking:
- other local anesthetics;
- strong painkillers, such as morphine or codeine;
- medicines used to treat irregular heartbeat (arrhythmia), such as lidocaine or mexiletine.
The doctor needs to know about these medicines to assess whether Ropivacaine BioQ can be given to the patient.
The patient should also inform their doctor if they are taking any of the following medicines:
- medicines used to treat depression (e.g., fluvoxamine);
- antibiotics used to treat bacterial infections (e.g., enoxacin).
This is because the removal of Ropivacaine BioQ from the body takes longer if the patient is taking these medicines.
If the patient is taking any of these medicines, they should avoid prolonged use of Ropivacaine BioQ.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
It is not known whether ropivacaine hydrochloride passes into breast milk.
As a precaution, the use of Ropivacaine BioQ should be avoided during pregnancy.
During treatment with Ropivacaine BioQ, breastfeeding should be temporarily stopped. The milk should be expressed and discarded during this time.
Driving and using machines
Ropivacaine BioQ may cause drowsiness and slowed reactions. After receiving Ropivacaine BioQ, the patient should not drive or operate machinery until the next day.
Ropivacaine BioQ contains sodium
The medicine contains 3.4 mg of sodium (the main component of common salt) per milliliter. This corresponds to 0.17% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use Ropivacaine BioQ
Ropivacaine BioQ will be administered to the patient by a doctor.
Ropivacaine BioQ will be administered by intravenous infusion to reduce pain after surgery. It will be administered as an infusion around a nerve (perineural) or into a surgical wound (infiltration anesthesia). For wound infiltration anesthesia, the doctor will place a catheter in the wound during surgery, which can be connected to the Ropivacaine ReadyfusOR infusion pump (also referred to as the "dosage device").
The infusion pump is a dosing device containing the infusion solution and equipped with a permanently attached tube with a connector that can be connected to the catheter placed in the wound or a port near the nerve.
The doctor or nurse will turn on the dosage device and connect it to the catheter/port. The patient does not need to do anything with the dosage device.
After the dosage device is turned on, it will continuously administer a fixed dose of the active substance, sufficient to relieve pain.
Warnings
- Avoid bending the tube, as this may result in an incorrect fluid flow rate.
- Do not tightly wrap the tube.
- Do not use the dosage device if any part of it is damaged or cracked, or if the connector on the tube appears to be broken, cracked, or damaged in any other way.
- The flow restrictor (a transparent rectangle) must remain attached to the patient's skin. Removing the tape or losing contact between the flow restrictor and the patient's skin may result in an incorrect fluid flow rate.
- Do not apply hot or cold compresses to the flow restrictor, as this may result in an incorrect fluid flow rate.
- In the event of accidental disconnection of the dosage device from the catheter/port during drug administration, do not reconnect it, as this may cause infection. Instead, contact the doctor or nurse and report the disconnection.
- The patient should not bathe or shower with the dosage device or while the catheter/port is still in place, as this may lead to infection.
- The patient should not disturb the wound dressings or the catheter/port, as this may lead to infection.
Using more than the recommended dose of Ropivacaine BioQ
Since the dosage device administers a fixed dose of the active substance continuously, serious side effects due to receiving more than the recommended dose of Ropivacaine BioQ are very unlikely.
If the dose is too high, the patient will need special treatment — the attending doctor is trained to handle such situations. The first symptoms of receiving more than the recommended dose of Ropivacaine BioQ are usually:
- dizziness or a feeling of emptiness in the head;
- numbness of the lips and the area around the mouth;
- numbness of the tongue;
- hearing problems;
- vision problems.
To minimize the risk of serious side effects, the doctor will stop administering Ropivacaine BioQ immediately if these symptoms occur. This means that if any of these symptoms occur in the patient or if the patient thinks they have received more than the recommended dose of Ropivacaine BioQ, they should immediately inform their doctor.
If the patient has any further doubts about using this medicine, they should consult their doctor or nurse.
4. Possible side effects
Like all medicines, Ropivacaine BioQ can cause side effects, although not everybody gets them.
Important side effects to look out for
Sudden life-threatening allergic reactions (such as anaphylaxis) are rare and occur in 1 to 10 patients out of 10,000. Possible symptoms include: sudden onset of rash, itching, or hives; swelling of the face, lips, tongue, or other parts of the body, and shortness of breath, wheezing, or difficulty breathing. If the patient notices that Ropivacaine BioQ is causing an allergic reaction, they should immediately inform their doctor.
Other possible side effects
Very common(symptoms may occur in more than 1 in 10 patients)
- Low blood pressure (hypotension) that may cause dizziness or a feeling of emptiness in the head.
- Nausea.
Common(symptoms may occur in up to 1 in 10 patients)
- Numbness.
- Dizziness.
- Headache.
- Slow or fast heartbeat (bradycardia, tachycardia).
- High blood pressure (hypertension).
- Vomiting.
- Difficulty urinating.
- High temperature (fever) and shivering (chills).
- Back pain.
Uncommon(symptoms may occur in up to 1 in 100 patients)
- Anxiety.
- Loss of skin sensation or sensitivity.
- Fainting.
- Breathing difficulties.
- Drop in body temperature (hypothermia).
- Some symptoms may occur when the patient receives more than the recommended dose of Ropivacaine BioQ (see also "Using more than the recommended dose of Ropivacaine BioQ" above). These include: seizures, dizziness or a feeling of emptiness in the head, numbness of the lips and the area around the mouth, numbness of the tongue, hearing problems, vision problems, speech problems, muscle stiffness, and shivering.
Rare(symptoms may occur in up to 1 in 1,000 patients)
- Heart attack (cardiac arrest).
- Irregular heartbeat (arrhythmia).
Unknown(frequency cannot be estimated from the available data)
- Involuntary muscle movements (dyskinesia).
Possible side effects observed with other local anesthetics that may also be caused by Ropivacaine BioQ
Rare(symptoms may occur in up to 1 in 1,000 patients)
- Nerve damage that may cause permanent problems.
Reporting side effects
If side effects occur, including any not listed in the leaflet, the patient should tell their doctor or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, PL-02 222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl . Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Ropivacaine BioQ
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label after "Expiry date". The expiry date refers to the last day of the month.
There are no special storage instructions for the medicine.
It is usually the doctor or hospital that stores Ropivacaine BioQ and is responsible for the quality of the product. The medicine should be visually inspected before use. It should only be used if it is clear and practically free from particles, and the container is not damaged.
The doctor or hospital is also responsible for the proper disposal of unused Ropivacaine BioQ.
6. Contents of the packaging and other information
What Ropivacaine BioQ contains
- The active substance of the medicine is ropivacaine hydrochloride (Ropivacaini hydrochloridum). One milliliter contains 2 mg of ropivacaine hydrochloride.
- The excipients are: sodium chloride, sodium hydroxide, or hydrochloric acid to adjust the pH, and water for injections.
What Ropivacaine BioQ looks like and what the pack contains
Ropivacaine BioQ is a clear, colorless solution for infusion.
The Ropivacaine ReadyfusOR infusion pump is an orange cylinder with black caps on both sides. It is designed to hold a transparent, collapsible bottle made of high-density polyethylene (HDPE) containing 250 ml of ropivacaine hydrochloride infusion solution. A permanently attached tube with a connector (Luer Lock type) is attached to it, which does not contain latex.
Each pack contains one Ropivacaine ReadyfusOR infusion pump and a case. Also available are sets that include a sterile, latex-free, fenestrated catheter for placement in the wound (6.5 or 15 cm long).
Marketing authorization holder and manufacturer
BioQ Pharma B.V.
Prins Bernhardplein 200
1097 JB Amsterdam
Netherlands
Manufacturer
BioQ Pharma B.V.
Prins Bernhardplein 200
1097 JB Amsterdam
Netherlands
Geryon Pharma Ltd
18 Owen Drive
Liverpool L24 1YL
United Kingdom
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria
Ropivacain ReadyfusOR 2 mg/ml Infusionslösung im Applikationssystem
Belgium
Ropivacaine Readyfusor 2 mg/ml solution pour perfusion en système d’administration
Croatia
Ropivakain BioQ Pharma 2 mg/ml otopina za infuziju u sustavu za primjenu
Czech Republic
Ropivacaine BioQ 2 mg/ml infuzní roztok v aplikačním systému
Denmark
Ropivacaine BioQ 2 mg/ml infusionsvæske, opløsning i administrationssystem
Finland
Ropivacaine BioQ 2 mg/ml infuusioneste, liuos, antovälineistö
France
Ropivacaine Readyfusor 2 mg/ml solution pour perfusion en système d’administration
Greece
Ropivacaine/ReadyfusOR 2 mg/ml διάλυμα για έγχυση σε σύστημα χορήγησης
Spain
Ropivacaína Readyfusor 2 mg/ml solución para perfusión en sistema de administración
Luxembourg
Ropivacaine ReadyfusOR 2 mg/ml solution pour perfusion en système d'administration
Norway
Ropivacaine BioQ 2 mg/ml infusjonsvæske, oppløsning i administreringssystem
Poland
Ropivacaine BioQ, 2 mg/ml, roztwór do infuzji w zestawie do podawania
Portugal
Ropivacaína BioQ 2 mg/ml solução para perfusão em sistema de administração
Slovakia
Ropivacaine Readyfusor 2 mg/ml infúzny roztok v aplikačnom systéme
Sweden
Ropivacaine BioQ 2 mg/ml infusionsvätska, lösning i administreringssats
United Kingdom (Northern Ireland)
Ropivacaine 2 mg/ml solution for infusion in administration system
Italy
Ropivacaina BioQ ReadyfusOR 2 mg/ml soluzione per infusione in sistema di somministrazione
Date of last revision of the leaflet: 12/10/2022.
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
The Ropivacaine BioQ medicinal product does not contain preservatives and is intended for single use only.
The solution should be visually inspected before use. It should only be used if it is clear and free from particles, and the container is not damaged.
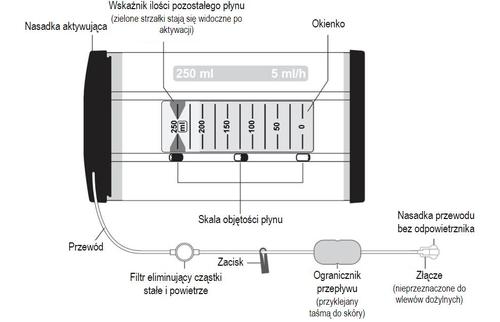
The Ropivacaine ReadyfusOR infusion pump
The Ropivacaine ReadyfusOR infusion pump (also referred to as the "dosage device") is a non-electric drug dispenser designed for bedside use.
The dosage device contains a collapsible bottle containing 250 ml of ropivacaine hydrochloride monohydrate infusion solution. A permanently attached tube with a connector (Luer Lock type) is attached to it. The tube, connector, and sterile fenestrated catheter (if included in the set, see section 6) do not contain latex.
For wound infiltration anesthesia, the fenestrated catheter should be placed in the wound during surgery in accordance with clinical guidelines specific to the location of the procedure. The catheter (if included in the set) ensures even distribution of the Ropivacaine BioQ medicinal product along the length of the wound in a 360° radius.
The fluid volume indicator is a set of green arrows indicating the amount of fluid remaining to be administered.
Instructions for use
- 1. Check the dosage device, flow restrictor, and tube for damage or tampering.
□ Check if the orange plug on the activation cap has been tampered with.
□ Check if the orange plug on the tube cap has been tampered with.
If damage or tampering is found, or if either of the plugs has been removed or tampered with, do not use this dosage device.
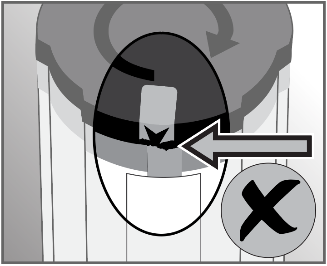
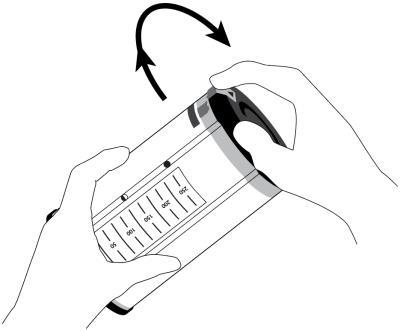
- 2. Start the fluid administration by turning the activation cap clockwise until the arrow on the orange plug is approximately aligned with the arrow on the label. Significant force is required. This is a normal situation that prevents accidental activation. The elements inside
the dosage device will move during activation.
Activation of the dosage device is indicated by the appearance of green arrows in the window indicating the amount of fluid remaining to be administered. Fluid flow can be seen above the filter within a few seconds, but the flow will be halted until the airless connector cap is removed.
- 3. Unscrew the tube cap to break the seal.
Check that the clamp is not tightened and ensure that fluid administration has started by observing fluid flow through the tube and flow restrictor.
After 1–2 minutes, the fluid will start to drip very slowly from the end of the tube.
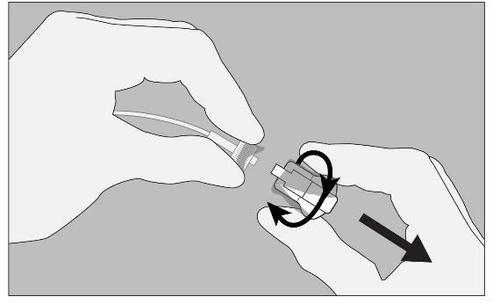
- 4. Connect the dosage device tube to the patient's port/catheter. Do not connect to an intravenous line.
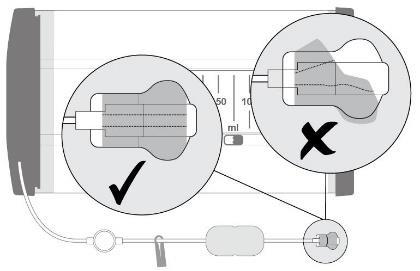
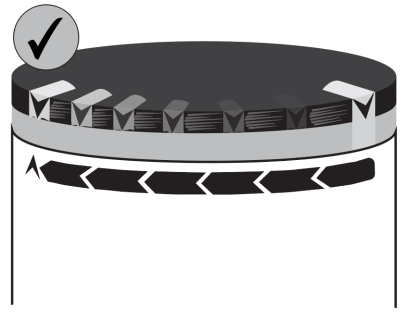
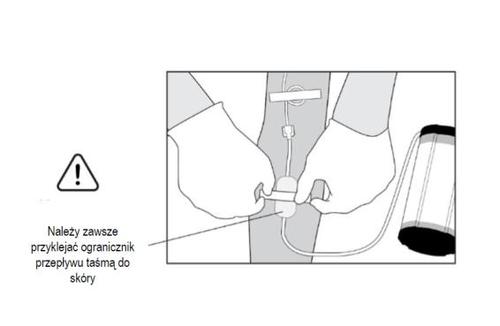
- 5. Attach the flow restrictor (a transparent rectangle) to the patient's skin using tape. The tape should be placed directly on the flow restrictor as shown in the illustration, away from the wound, avoiding pulling on the tube and disturbing the position of the catheter/port. Secure the tube and connectors with tape at the end.
Warning: the flow restrictor must remain attached and in contact with the patient's skin. Loss of
contact may result in an incorrect
fluid flow rate.
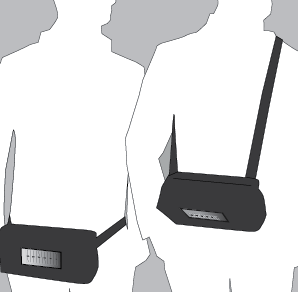
- 6. Place the dosage device in the provided case. The case can be worn by the patient over the shoulder or around the waist like a belt.
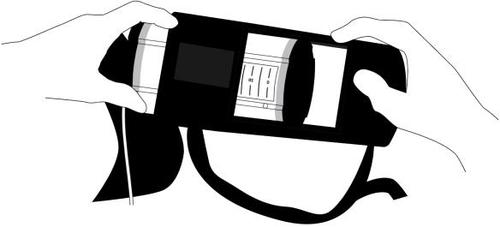
To prevent the catheter/port from being pulled out, it is recommended that the case with the dosage device be attached to the patient's body at all times.
- 7. Fluid administration can be observed in the dosage device window. The dosage device delivers approximately 5 ml of fluid per hour.
The green arrows in the window indicate the amount of fluid remaining in the dosage device (in ml).
Periodically monitor the position of the fluid volume indicators to check for excessive flow rate. For information on symptoms of overdose, see "Using more than the recommended dose of Ropivacaine BioQ" (section 3).
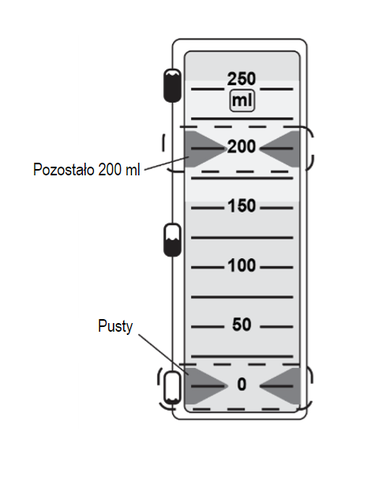
- 8. Administration is complete when the single-dose container is empty, as indicated by the green arrows showing zero in the window.
- 9. After administration is complete, remove the dosage device from the patient.
- 10. After use, discard the empty dosage device, including any remaining unused solution, in accordance with local requirements.
Warnings
- The dosage device is for single use only. Do not reuse or reconnect the dosage device.
- The dosage device cannot be sterilized in an autoclave. The fluid path in the dosing system has been sterilized.
- Do not connect the dosage device to an intravenous line.
- Avoid bending the tube, as this may result in an incorrect fluid flow rate.
- Do not tightly wrap the tube.
- Do not use the dosage device if any part of it is damaged or cracked, or if the connector on the tube appears to be broken, cracked, or damaged in any other way.
- The flow restrictor (a transparent rectangle) must remain attached to the patient's skin. Removing the tape or losing contact between the flow restrictor and the patient's skin may result in an incorrect fluid flow rate.
- Do not apply hot or cold compresses to the flow restrictor, as this may result in an incorrect fluid flow rate.
- In the event of accidental disconnection of the dosage device from the catheter/port during drug administration, do not reconnect it, as this may cause infection. Instead, contact the doctor or nurse and report the disconnection.
- The patient should not bathe or shower with the dosage device or while the catheter/port is still in place, as this may lead to infection.
- The patient should not disturb the wound dressings or the catheter/port, as this may lead to infection.
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterBioQ Pharma B.V. Copea Pharma Europe Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ropivacaine BioqDosage form: Solution, 2 mg/mlActive substance: ropivacainePrescription not requiredDosage form: Solution, 10 mg/mlActive substance: ropivacainePrescription not requiredDosage form: Solution, 2 mg/mlActive substance: ropivacainePrescription not required
Alternatives to Ropivacaine Bioq in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ropivacaine Bioq in Spain
Alternative to Ropivacaine Bioq in Ukraine
Online doctors for Ropivacaine Bioq
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ropivacaine Bioq – subject to medical assessment and local rules.














