ROPIVACAINE READYFUSOR 2 mg/mL SOLUTION FOR INFUSION IN ADMINISTRATION SYSTEM

How to use ROPIVACAINE READYFUSOR 2 mg/mL SOLUTION FOR INFUSION IN ADMINISTRATION SYSTEM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ropivacaína Readyfusor 2 mg/ml solution for infusion in administration system
ropivacaína, hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Ropivacaína Readyfusor is and what it is used for
- What you need to know before you use Ropivacaína Readyfusor
- How to use Ropivacaína Readyfusor
- Possible side effects
- Storage of Ropivacaína Readyfusor
- Contents of the pack and other information
1. What Ropivacaína Readyfusor is and what it is used for
The name of your medicine is “Ropivacaína Readyfusor 2 mg/ml solution for infusion in administration system”. It contains the active substance called ropivacaína hydrochloride which belongs to a class of medicines called local anesthetics.
Ropivacaína Readyfusor is used in adults for the treatment of acute pain. It numbs (anesthetizes) a part of the body, for example, after surgery.
2. What you need to know before you use Ropivacaína Readyfusor
Do not use Ropivacaína Readyfusor
- if you are allergic to ropivacaína hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to any other local anesthetic of the same class (such as lidocaine or bupivacaine).
- if you have a reduced blood volume (hypovolemia).
- to inject it into a blood vessel, spinal column or joint to numb a specific area of your body, or into the neck of the uterus to relieve pain during childbirth.
If you are not sure if any of the above applies to you, tell your doctor before using Ropivacaína Readyfusor.
Warnings and precautions
Tell your doctor or nurse before using Ropivacaína Readyfusor, especially:
- if you have heart, liver or kidney problems
- if you have a rare blood pigment disease called “porphyria” or if someone in your family has it, as your doctor may need to use a different medicine
- if you have any other illness or medical condition.
Using Ropivacaína Readyfusor with other medicines
Tell your doctor if you are using or have recently used other medicines. This is because Ropivacaína Readyfusor may affect the action of some medicines and some medicines may have an effect on Ropivacaína Readyfusor.
In particular, tell your doctor if you are taking any of the following medicines:
- Other local anesthetics
- Strong painkillers, such as morphine or codeine
- Medicines used to treat irregular heartbeats (arrhythmia), such as lidocaine and mexiletine.
Your doctor should know that you are using these medicines to decide if Ropivacaína Readyfusor can be given to you.
Tell your doctor if you are taking any of the following medicines:
- Medicines used to treat depression (e.g. fluvoxamine)
- Antibiotics to treat bacterial infections (e.g. enoxacin).
This is because your body needs more time to eliminate Ropivacaína Readyfusor if you are taking these medicines.
If you are taking any of these medicines, you should avoid prolonged use of Ropivacaína Readyfusor.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
It is not known if ropivacaína hydrochloride affects pregnancy or if it passes into breast milk.
As a precaution, it is recommended to avoid using Ropivacaína Readyfusor during pregnancy.
During treatment with Ropivacaína Readyfusor, breastfeeding should be temporarily stopped. During this period, milk should be expressed and discarded.
Driving and using machines
Ropivacaína Readyfusor may make you feel drowsy and affect your reaction speed. After using Ropivacaína Readyfusor, you should not drive or use tools or machines until the next day.
Ropivacaína Readyfusor contains sodium
This medicine contains 3.4 mg of sodium (main component of cooking/table salt) per milliliter. This is equivalent to 0.17% of the maximum recommended daily sodium intake for an adult.
3. How to use Ropivacaína Readyfusor
Ropivacaína Readyfusor will be given to you by a doctor.
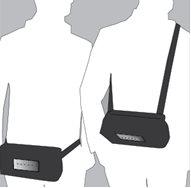
Ropivacaína Readyfusor will be administered by infusion to reduce pain after surgery. It will be administered near a nerve (perineurally) or in a surgical wound (infiltration). For wound infiltration, your doctor will place a catheter in the wound during surgery, which can be connected to the Ropivacaína Readyfusor infusion pump (hereinafter referred to as the “dispenser”).
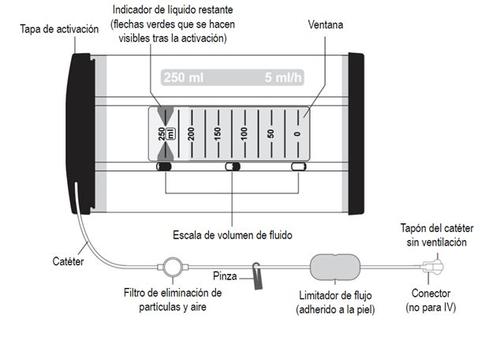
The dispenser is a device that contains the infusion solution and has a catheter with a permanent connector that can be connected to the catheter placed in the wound or near the nerve.
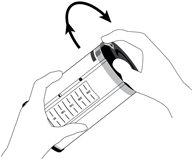
Your doctor or nurse will activate the dispenser and connect it to the catheter/access route. You will not have to do anything with the dispenser.
After activation, the dispenser will continuously administer a defined dose of the active substance, sufficient for pain relief.
Warnings
- Avoid twisting the catheter, as this could cause inadequate liquid flow rate.
- Do not wrap the catheter with any wrapping.
- Do not use the dispenser if any part is damaged or cracked or if the catheter connector appears broken, cracked or damaged in any way.
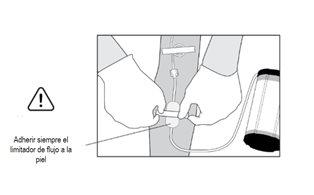
- The flow limiter (transparent rectangle) should remain stuck to your skin. Removing the adhesive tape or allowing the flow limiter to lose contact with the skin may cause inadequate liquid flow rate.
- Do not place hot or cold compresses on the flow limiter, as this may cause inadequate liquid flow rate.
- Do not reconnect the dispenser if it is accidentally disconnected from the catheter/access route during medication administration, as this could cause an infection. Inform your doctor or nurse if the dispenser has been disconnected.
- Do not bathe or shower with the dispenser or while the catheter/access route is still in place, as this could cause an infection.
- Do not manipulate the wound dressings or the catheter/access route, as this could cause an infection.
If you use more Ropivacaína Readyfusor than you should
Since the dispenser continuously administers a defined dose of the active substance, it is very unlikely that side effects will occur as a result of using more Ropivacaína Readyfusor than you should.
If the dose received is too high, you will need special treatment and the doctor treating you is trained to handle these situations. The first symptoms that you have been given too much Ropivacaína Readyfusor are usually related to:
- dizziness or fainting
- numbness of the lips and around the mouth
- numbness of the tongue
- hearing problems
- vision problems
To reduce the risk of serious side effects, your doctor will stop the administration of Ropivacaína Readyfusor as soon as these signs appear. This means that if you experience any of these symptoms or think you may have received too much Ropivacaína Readyfusor, tell your doctor immediately.
If you have any further questions on the use of this product, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Important side effects to look out for
Rare, sudden-onset allergic reactions that are life-threatening (such as anaphylaxis, including anaphylactic shock) and affect 1 to 10 patients in 10,000. Possible symptoms include sudden onset rash, itching or hives, swelling of the face, lips, tongue or other parts of the body, shortness of breath, wheezing or difficulty breathing, and a feeling of loss of consciousness. If you think Ropivacaína Readyfusor is causing an allergic reaction, tell your doctor immediately.
Other possible side effects
Very common(may affect more than 1 in 10 patients)
- Low blood pressure (hypotension). This may make you feel dizzy or faint.
- Feeling sick (nausea)
Common(may affect up to 1 in 10 patients)
- Tingling
- Feeling dizzy
- Headache
- Slow or fast heart rate (bradycardia, tachycardia)
- High blood pressure (hypertension)
- Feeling sick (vomiting)
- Difficulty urinating
- High temperature (fever) or chills
- Back pain
Uncommon(may affect up to 1 in 100 patients)
- Anxiety
- Decreased sensitivity or feeling in the skin
- Fainting
- Breathing difficulties
- Low body temperature (hypothermia)
- Some symptoms may appear if you have been given too much Ropivacaína Readyfusor (see also the previous section “If you use more Ropivacaína Readyfusor than you should”). These include seizures (convulsions), feeling dizzy or faint, numbness of the lips and around the mouth, numbness of the tongue, hearing problems, vision problems, speech problems, muscle stiffness and trembling.
Rare(may affect up to 1 in 1,000 patients)
- Heart attack (cardiac arrest)
- Irregular heartbeat (arrhythmias)
Frequency not known(frequency cannot be estimated from the available data)
- Involuntary muscle movements (dyskinesia)
Possible side effects observed with other local anesthetics that may also be caused by Ropivacaína Readyfusor include
Rare(may affect up to 1 in 1,000 patients)
- Nerve damage. This can cause permanent problems.
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ropivacaína Readyfusor
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after “EXP”. The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
Normally, your doctor or hospital will store Ropivacaína Readyfusor and are responsible for the quality of the product. The medicine should be inspected visually before use. The solution should only be used if the solution is clear, practically free of particles and if the packaging is intact.
They are also responsible for disposing of any unused Ropivacaína Readyfusor correctly.
6. Container Contents and Additional Information
Composition of Ropivacaína Readyfusor
- The active ingredient is ropivacaine hydrochloride. Each ml contains 2 mg of ropivacaine hydrochloride.
- The other components are sodium chloride, sodium hydroxide solution or hydrochloric acid (for pH adjustment) and water for injectable preparations.
Appearance of the Product and Container Contents
Ropivacaína Readyfusor is a clear, colorless solution for infusion.
Ropivacaína Readyfusor infusion pump is an orange cylinder with black caps on each side. It is designed to contain a high-density polyethylene (HDPE) transparent bellows bottle with 250 ml of ropivacaine hydrochloride monohydrate solution for infusion. A catheter with a connector (luer lock) that does not contain latex is permanently attached to it.
Each container contains a Ropivacaína Readyfusor infusion pump and a transport bag. There are also containers that include a sterile latex-free multiperforated catheter (6.5 or 15 cm in length) for placement in the wound.
Marketing Authorization Holder
BioQ Pharma B.V.
Basisweg 10
1043 AP Amsterdam
Netherlands
Manufacturer
BioQ Pharma B.V.
Basisweg 10
1043 AP Amsterdam
Netherlands
Copea Pharma Europe Limited
Unit 2, Medici House, Ashbourne Manufacturing Park
Ashbourne, Co. Meath A84 KH58
Ireland
Local Representative
Euromed Pharma Spain
Av. Eduard Maristany 430-432
08918, Badalona (Barcelona)
Spain
This Medicinal Product is Authorized in the Member States of the European Economic Areaand in the United Kingdom (Northern Ireland) under the Following Names:
Austria | Ropivacain ReadyfusOR 2 mg/ml Infusionslösung im Applikationssystem |
Belgium | Ropivacaine Readyfusor 2 mg/ml solution pour perfusion en système d’administration |
Denmark | Ropivacaine BioQ 2 mg/ml infusionsvæske, opløsning i administrationssystem |
Slovakia | Ropivacaine Readyfusor 2 mg/ml infúzny roztok v aplikacnom systéme |
Spain | Ropivacaína Readyfusor 2 mg/ml solución para perfusión en sistema de administración |
Finland | Ropivacaine BioQ 2 mg/ml infuusioneste, liuos, antovälineistö |
France | Ropivacaine Readyfusor 2 mg/ml solution pour perfusion en système d’administration |
Italy | Ropivacaina BioQ ReadyfusOR 2 mg/ml soluzione per infusione in sistema di somministrazione |
Luxembourg | Ropivacaine ReadyfusOR 2 mg/ml solution pour perfusion en système d'administration |
Norway | Ropivacaine BioQ 2 mg/ml infusjonsvæske, oppløsning i administreringssystem |
Poland | Ropivacaine BioQ, 2 mg/ml, roztwór do infuzji w zestawie do podawania |
Portugal | Ropivacaína BioQ 2 mg/ml solução para perfusão em sistema de administração |
United Kingdom (Northern Ireland) | Ropivacaine 2 mg/ml solution for infusion in administration system |
Czech Republic | Ropivacaine BioQ 2 mg/ml infuzní roztok v aplikacním systému |
Sweden | Ropivacaine BioQ 2 mg/ml infusionsvätska, lösning i administreringssats |
Date of Last Revision of this Leaflet:09/2023
--------------------------------------------------------------------------------------------------------------------
This Information is Intended Only for Doctors or Healthcare Professionals:
Ropivacaína Readyfusor does not contain preservatives and is intended for single use.
The solution should be visually inspected before use. The solution must only be used if the solution is clear, practically free of particles, and if the container is intact.
Ropivacaína Readyfusor Infusion Pump
Ropivacaína Readyfusor infusion pump (hereinafter referred to as "dispenser") is a non-electric medication dispenser designed for use in the healthcare setting. All necessary materials for medication administration are included.
The dispenser contains a bellows bottle with 250 ml of ropivacaine hydrochloride monohydrate solution for infusion. A catheter with a connector (luer lock) is permanently attached. Neither the catheter nor the connector nor the sterile multiperforated catheter (when included in the container, see section 6) contains latex.
For wound infiltration, a multiperforated catheter will be placed in the wound during surgery according to the specific guidelines of the procedure location. The catheter (when included in the container) distributes Ropivacaína Readyfusor uniformly along the length of the wound in a 360° radius.
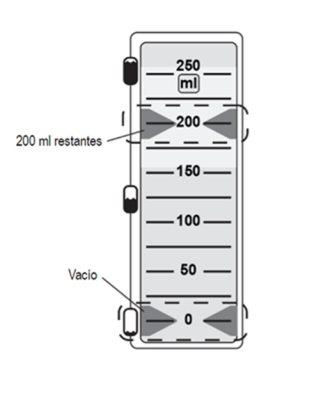
The liquid remaining indicator is a pair of green arrows indicating the amount of liquid left to be administered.
|
Instructions for Use
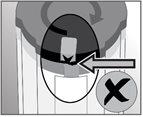
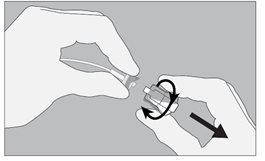
Check that the orange adhesive seal on the activation cap is intact. Check that the orange adhesive seal on the catheter plug is intact. Do not use the dispenser if damage is observed or if there is no seal or if it is damaged. |
|
The dispenser is activated when the green arrows of the liquid remaining indicator are visible in the window. The liquid flow can be seen upstream of the filter within seconds, but the flow will stop until the non-ventilated cap is removed. |
|
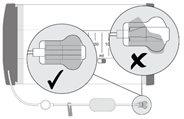
Check that the clamp is not engaged and ensure that liquid dispensing has started by observing that liquid flows through the catheter and flow limiter. After 1-2 minutes, the liquid will start to drip very slowly from the end of the catheter. |
|
| |
Warning: The flow limiter must remain attached to the patient's skin. If contact is lost, inadequate liquid dispensing speed may occur.
|
|
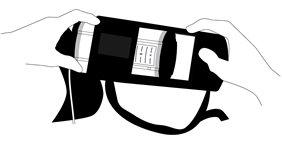
To prevent the catheter/access site from coming out, it is recommended to keep the bag attached to the patient with the dispenser inside at all times.
|
|
| |
| |
|
Warnings
- The dispenser is intended for single use. Do not reuse or reconnect the dispenser.
- The dispenser must not be sterilized in an autoclave. The fluid path in the dispensing system has already been sterilized.
- The dispenser must not be connected to an IV catheter.
- Twisting the catheter should be avoided, as this could cause inadequate liquid dispensing speed.
- Do not encircle the catheter with any wrapping.
- Do not use the dispenser if any part is damaged or cracked or if the catheter connector appears broken, cracked, or damaged in any way.
- The flow limiter (transparent rectangle) must remain attached to the patient's skin. Removing the adhesive tape or allowing the flow limiter to lose contact with the skin may cause inadequate liquid dispensing speed.
- Do not place hot or cold compresses over the flow limiter, as this may cause inadequate liquid dispensing speed.
- The dispenser must not be reconnected if it is accidentally disconnected from the catheter/access site during medication administration, as this could cause an infection.
- The patient must not bathe or shower with the dispenser or while the catheter/access site remains in place, as this could cause an infection.
- The patient must not manipulate the wound dressings or the catheter/access site, as this could cause an infection.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROPIVACAINE READYFUSOR 2 mg/mL SOLUTION FOR INFUSION IN ADMINISTRATION SYSTEMDosage form: INJECTABLE, 100 mgActive substance: ropivacaineManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE PERFUSION, 2 mg/mlActive substance: ropivacaineManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE, 75 mgActive substance: ropivacaineManufacturer: Altan Pharmaceuticals SaPrescription required
Online doctors for ROPIVACAINE READYFUSOR 2 mg/mL SOLUTION FOR INFUSION IN ADMINISTRATION SYSTEM
Discuss questions about ROPIVACAINE READYFUSOR 2 mg/mL SOLUTION FOR INFUSION IN ADMINISTRATION SYSTEM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions