
Relenza

Ask a doctor about a prescription for Relenza

How to use Relenza
Leaflet accompanying the packaging: information for the user
Relenza
5 mg/dose, inhalation powder, divided
Zanamivir
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for a specific person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist, or nurse.See section 4.
Table of contents of the leaflet
- 1. What is Relenza and what is it used for
- 2. Important information before taking Relenza
- 3. How to take Relenza
- 4. Possible side effects
- 5. How to store Relenza
- 6. Contents of the pack and other information
1. What is Relenza and what is it used for
Relenza contains zanamivir, which belongs to a group of medicines called antivirals.
Relenza is used to treat influenza(influenza virus infection), it reduces the severity of symptoms and accelerates recovery.
Relenza is also used to prevent influenza during an epidemic.
Relenza can be used in adults and children over 5 years of age.
Relenza is administered by inhalation (inhalation) into the lungs, as absorption after swallowing is weak. The influenza virus infects the lungs; when Relenza is inhaled, it acts directly on the viruses inside the lungs.
Relenza does not replace influenza vaccination.You should consult your doctor about whether you need to receive an influenza vaccine.
2. Important information before taking Relenza
When not to take Relenza
- If the patient is allergicto zanamivir or any of the other ingredients of this medicine (listed in section 6),
- If the patient is allergic to milk protein(see "Relenza contains lactose and milk protein" and section 6).
Do not take Relenza in children under 5 years of age.
When to exercise special caution when taking Relenza
- If the patient experiences a feeling of constriction in the throat or chest while taking Relenza.Very rarely, Relenza may cause reactions including:
- a feeling of constriction in the throat and chest,
- difficulty breathing.If any of these symptoms occur while taking Relenza:
- stop taking Relenza and seek medical attention immediately,
- contact your doctor or the emergency department of the nearest hospital.
- If the patient has asthma or other lung or breathing problems.If the patient has:
- asthma,
- other lung diseases that cause breathing difficulties - such as emphysema, chronic obstructive pulmonary disease (COPD) or chronic bronchitis.Tell your doctor before taking Relenza, as they will be able to monitor your condition more closely.
- If the patient is taking inhaled medicinesfor asthma or other breathing disorders, they should read the following part of this leaflet carefully (Using Relenza with inhaled medicines for breathing disorders) before taking Relenza.
Using Relenza with inhaled medicines for breathing disorders
If the patient is taking inhaled medicinesfor asthma or other breathing disorders, they should continue to take them at the usual times.
If Relenza is prescribed at the same time as other inhaled medicines, take these inhaled medicines a few minutes before taking Relenza.
Make sure an inhaler with a fast-acting bronchodilator
(such as salbutamol) is at hand while taking Relenza.
Relenza and other medicines
Tell your doctor or pharmacist about all medicines you are taking
now or recently, and about any medicines you plan to take, including those available without a prescription.
If you are taking inhaled medicinesfor asthma or breathing disorders, make sure you have read the above warnings.
If the patient has been prescribed an influenza vaccine
Vaccination can be performed at any time, even if the patient is taking Relenza, which helps prevent influenza.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before taking this medicine.
There is only limited data on the safety of Relenza in pregnant women. Although there is no evidence that Relenza harms the fetus, it should not be taken during pregnancy unless prescribed by a doctor.
The active substance (zanamivir) may pass into breast milk, so breastfeeding should be avoided while taking Relenza. Consult your doctor - they will decide whether to stop/discontinue treatment with Relenza or stop breastfeeding, depending on what is best for the patient and the child.
Driving and using machines
Relenza is unlikely to affect the ability to drive or use machines.
Relenza contains lactose and milk protein.
Relenza contains a sugar called lactose and milk protein. If the patient has previously been diagnosed with intolerance to some sugars, they should consult their doctor before taking Relenza.
3. How to take Relenza
This medicine should always be taken as directed by your doctor or pharmacist.If you are unsure, consult your doctor or pharmacist.
Relenza is a powder that is inhaled into the lungs through the mouth, using the Diskhaler inhalation device.
The Rotadisk blister pack, which is placed in the Diskhaler inhalation device, contains 4 doses of powder.
The powder in the Rotadisk blister pack can only be used with the Diskhaler inhalation device.
Do not take Relenza in children under 5 years of age.
When to start taking Relenza
Treating influenza
The best results are obtained when treatment is started as soon as possible after symptoms appear:
- In adults, within 48 hoursof symptom onset;
- In children, within 36 hoursof symptom onset.
Preventing influenza
If someone in the household has influenza, to help prevent illness, start taking Relenza as soon as possibleafter contact with that person:
- In adults and children, within 36 hoursof contact with the infected person.
If there is an influenza epidemic in the area,follow your doctor's advice on when to start taking Relenza.
Dosage of Relenza
The dosage depends on whether Relenza is being used to treat or prevent influenza.
Treating influenza
Adults and children (5 years and older):usually take 2 inhalations (2 doses) 2 times a day for 5 days.
Preventing influenza
If someone in the household has influenza
Adults and children (5 years and older):usually take 2 inhalations (2 doses) once a day for 10 days.
If there is an influenza epidemic in the area
Adults and children (5 years and older):take 2 inhalations (2 doses) once a day for up to 28 days.
Taking a higher dose of Relenza than recommended
Accidental overdose of Relenza is unlikely to cause problems. However, if the patient is concerned or does not feel well, especially if they have asthma or other lung problems, they should tell their doctor.
Missing a dose of Relenza
If a dose of Relenza is missed, take it as soon as possible and continue with the treatment as before.
Do not take a double dose to make up for a missed dose.
Stopping Relenza treatment
During influenza treatment, it is important to complete the full course(usually 5 days), even if the patient feels better. Otherwise, influenza symptoms may return.
If the patient wants to stop taking Relenza earlier:
they should consult their doctor for advice.
The other side of this leaflet shows the step-by-step instructions for using the Diskhaler device.
Read these instructions before taking the first dose of Relenza. If the patient is unsure how to use the Diskhaler device, they should consult their pharmacist and review the instructions together.
4. Possible side effects
Like all medicines, Relenza can cause side effects, although not everybody gets them.
Side effects to look out for
Severe allergic reactions
Occur less often than 1 in 1000people taking Relenza. Symptoms include:
- hives and itching;
- swelling, sometimes of the face, lips, or throat, causing breathing difficulties;
- fainting.
If any of these symptoms occur:
- seek medical attention immediately.
Severe skin reactions
These side effects are very rare and may occur less often than 1 in 1000people taking Relenza:
- A skin rash that may be blistering and looks like small targets (dark spots in the center, surrounded by a lighter area with a dark ring around it) - erythema multiforme.
- A widespread rash with blisters and peeling skin, especially around the mouth, nose, eyes, and genitals - Stevens-Johnson syndrome.
- Widespread peeling of the skin, covering a large area of the body - toxic epidermal necrolysis. If any of these symptoms occur, seek medical attention immediately. Stop taking Relenza.
Other common side effects
Occur less often than 1 in 10people taking Relenza:
- Skin rash
Other uncommon side effects
Occur less often than 1 in 100people taking Relenza:
Feeling of constriction in the throat and chest, shortness of breath, or sudden difficulty breathing.
Difficulty breathing.In patients with lung diseases (such as asthma or COPD), it may be necessary to monitor them closely while taking Relenza, due to the possibility of these side effects.
- Swelling of the face, mouth, and throat.
- Hives (itching bumps on the skin).
- Fainting and dizziness.If the patient does not feel well while taking Relenza, they may faint or feel dizzy after inhaling Relenza. Before inhaling, the patient should sit in a comfortable position, and after inhaling the dose, they should hold their breath only for as long as it is comfortable for them. If the patient does not feel well, it is recommended that they take the dose of Relenza in the presence of another person.
If any of these symptoms occur while taking Relenza:
stop taking the medicine and seek medical attention immediately- contact your doctor or the emergency department of the nearest hospital.
Sudden changes in behavior, hallucinations, and seizures.
While taking Relenza, changes in behavior, such as feeling disoriented or unresponsive, have occurred. Some patients may also experience hallucinations (seeing, hearing, or feeling things that are not real) or seizures, which can lead to loss of consciousness. Parents should be especially aware of these symptoms in their children (young or adolescent) with influenza. These symptoms have also been observed in people with influenza who were not taking Relenza. It is not known whether Relenza had an effect on their occurrence.
If any of these symptoms occur
- seek medical attention immediately.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. By reporting side effects, more information can be gathered on the safety of the medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store Relenza
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after "EXP".
The expiry date refers to the last day of the month stated. The abbreviation "Lot" means the batch number of the product.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
Ask your pharmacist how to dispose of the Diskhaler inhalation device that is no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Relenza contains
The active substance is zanamivir (5 mg per dose).
The other ingredient is lactose monohydrate (which contains milk protein).
What Relenza looks like and contents of the pack
Four doses of Relenza powder are in a silver-colored foil blister pack called Rotadisk. Each dose contains 5 mg of zanamivir.
The powder is inhaled through the mouth using a plastic inhalation device called Diskhaler.
Relenza is available in two pack sizes:
- Single-day pack,containing one Relenza Rotadisk blister pack and one Diskhaler;
- Five-day treatment pack,containing five Relenza Rotadisk blister packs and one Diskhaler.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
D24 YK11
Ireland
Manufacturer
Glaxo Wellcome Production
Zone Industrielle No.2
23 Rue Lavoisier
27000 Evreux
France
GlaxoSmithKline Trading Services Limited
12 Riverwalk,
Citywest Business Campus
Dublin 24
Ireland
Manufacturer of the Diskhaler inhalation device
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Irlandia
To obtain more detailed information, please contact the representative of the marketing authorization holder:
GSK Services Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warszawa
tel. (22) 576-90-00
Date of last revision of the leaflet:January 2025
--------------------------------------------------------------------------------------------------------------------------
The Diskhaler device consists of three parts:
Do not disassemble the Diskhaler device before reading the instructions.
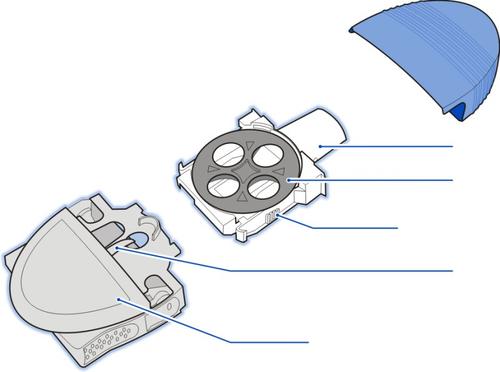
The Rotadisk blister pack is designed for the Diskhaler device
Place the Rotadisk on the Diskhaler device's carousel.
The Rotadisk contains four blisters. Each blister contains 5 mg of zanamivir. The standard dose is two blisters (10 mg).
Warning:
Do not puncture the blister pack before placing it in the Diskhaler device. The blister pack can remain in the Diskhaler device at all times, but it should only be punctured immediately before use.
Keep the Diskhaler device clean. After use, wipe the mouthpiece with a cloth. When not in use, cover the mouthpiece with the blue cap.
If you experience any problems with the Diskhaler device or the Rotadisk blister pack, contact your doctor or pharmacist.
STEP-BY-STEP INSTRUCTIONS FOR USING THE DISKHALER DEVICE
INTRODUCTION OF THE ROTADISK INTO THE DISKHALER DEVICE:
1
Remove the blue mouthpiece cap. Make sure the mouthpiece inside and outside is clean.
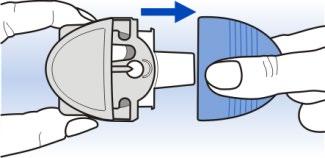
Hold the Diskhaler device by the edges of the body, as shown in the picture, and slide the white mouthpiece tray out to the stop.
2
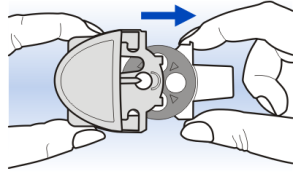
3 Gently pressing the tabs on either side of the white mouthpiece tray, remove the carousel from the Diskhaler device body.
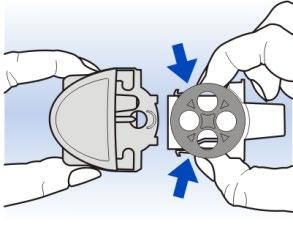
The white mouthpiece tray should come out easily.
4 Remove the Rotadisk from its packaging and place it on the carousel.
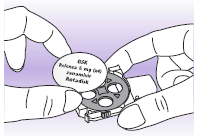
Make sure the printed side is facing up, and the blisters are facing down. The blisters "fit" into the holes in the carousel.
5 Slide the white mouthpiece tray back into the Diskhaler device body.
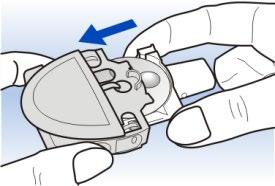
If you are not ready to inhale, replace the blue mouthpiece cap.
RELEASING THE DOSE:
Do not perform the following steps if you are not ready to inhale.
6 Hold the Diskhaler device horizontally.
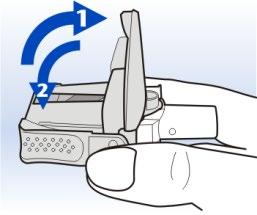
Hold the Diskhaler device horizontally
Lift the lid of the body to the stop, to the vertical position.
The lid must be in the vertical position to puncture the blister pack from both the top and bottom.
Close the lid of the body.
The Diskhaler device is now ready for use. Hold it horizontally until you have finished inhaling the dose.
If you are taking other inhaled medicines,read section 2 of this leaflet carefully Using Relenza with inhaled medicines for breathing disorders.
INHALING THE DOSE:
7
Before putting the Diskhaler device mouthpiece in your mouth. Exhale deeply, holding the Diskhaler device away from your mouth. Do not blow into the Diskhaler device. If you do, the powder will be blown out of the Rotadisk.
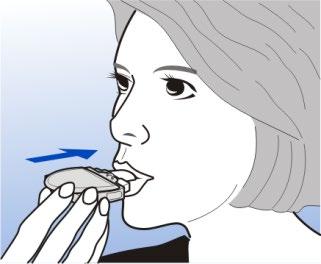
Hold the Diskhaler device horizontally
Hold the Diskhaler device horizontally, put the mouthpiece in your mouth, and close your lips tightly around it.
Do not bite the mouthpiece. Do not cover the air intake holes on either side of the mouthpiece.
Take a deep, rapid breath in through the mouthpiece, and hold your breath for a few seconds.
Remove the Diskhaler device from your mouth.
Hold your breathfor a few more seconds, as long as you can.
Preparing the Diskhaler device for reuse(second part of the dose):
8 Slide the white mouthpiece tray out to the stop, then slide it back in.
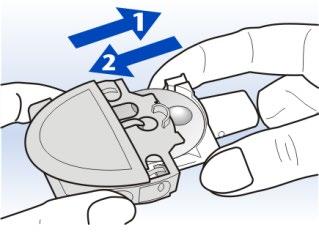
This will rotate the Rotadisk.
Repeat this step until a full blister is in place under the piercing needle.
To take the next inhalation, follow steps 6-7.
9
After taking the full dose (usually two inhalations):
Wipe the mouthpiece with a cloth and replace the blue mouthpiece cap.It is essential to keep the Diskhaler device clean.
Replacing the Rotadisk in the Diskhaler device:
10
If all four blisters are empty, replace the Rotadisk blister pack in the Diskhaler device and insert a new one, following steps 1-5.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlaxo Wellcome Production GlaxoSmithKline Trading Services Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RelenzaDosage form: Capsules, 30 mgActive substance: oseltamivirPrescription requiredDosage form: Capsules, 45 mgActive substance: oseltamivirPrescription requiredDosage form: Capsules, 75 mgActive substance: oseltamivirPrescription required
Alternatives to Relenza in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Relenza in Spain
Alternative to Relenza in Ukraine
Online doctors for Relenza
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Relenza – subject to medical assessment and local rules.














