
Pulveril
Ask a doctor about a prescription for Pulveril

How to use Pulveril
PATIENT INFORMATION LEAFLET: USER INFORMATION
Pulveril, 25 micrograms/dose, inhalation aerosol, suspension
Salmeterol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this leaflet, you may need to read it again.
In case of any doubts, consult a doctor or pharmacist.
This medicine has been prescribed specifically for you. Do not pass it on to others.
The medicine may harm another person, even if their symptoms are the same.
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What Pulveril is and what it is used for
- 2. Important information before using Pulveril
- 3. How to use Pulveril
- 4. Possible side effects
- 5. How to store Pulveril
- 6. Contents of the pack and other information
1. WHAT PULVERIL IS AND WHAT IT IS USED FOR
Pulveril contains the active substance salmeterol. It is a long-acting bronchodilator. It helps to maintain the dilation of the airways, making it easier to breathe in and out.
The effect usually starts within 10 to 20 minutes and lasts for 12 hours or longer.
The doctor prescribes Pulveril to prevent breathing difficulties. The cause may be asthma. Regular use of Pulveril will prevent asthma attacks, including those caused by physical exertion or nighttime asthma symptoms.
Regular use of Pulveril will also help prevent breathing difficulties caused by other chest diseases, such as chronic obstructive pulmonary disease (COPD).
Pulveril helps prevent shortness of breath and wheezing, but it is not effective when these symptoms have already occurred. In such cases, a rapidly acting bronchodilator, such as salbutamol, should be used.
Pulveril is contained in an inhaler. The medicine in the form of an aerosol is inhaled through the mouth directly into the lungs.
Pulveril contains norflurane. The medicine does not contain any freon gas. Inhalation medicines containing norflurane are less harmful to the environment than older medicines containing freon gas. The taste of Pulveril may be different from that of previously used freon-containing medicines, but it does not affect its action.
If the patient is being treated for asthma, they should always receive Pulveril in the form of an inhalation and an inhaled steroid (or in rare cases, a steroid in tablets).
Both medicines should be used regularly.
2. IMPORTANT INFORMATION BEFORE USING PULVERIL
When not to use Pulveril
if the patient is allergic (hypersensitive) to salmeterol xinafoate or any of the other ingredients of the medicine.
if the patient is allergic to peanuts or soy, they should not use Pulveril, as it contains soy lecithin.
When to be cautious when using Pulveril
If asthma symptoms or breathing difficulties worsen, the patient should immediately consult their doctor.
doctor. This may be manifested by wheezing, a feeling of tightness in the chest, or a greater than usual need for a rapidly acting bronchodilator. In such cases, the patient should not increase the number of Pulveril inhalations, as their condition may worsen, leading to severe illness. The patient should contact their doctor, as a change in asthma treatment may be necessary.
If the doctor has prescribed Pulveril for asthma, the patient should continue to take their previously used asthma medicines, such as inhaled steroids or steroids in tablets. They should use them in the same doses as before, unless the doctor recommends a change.
The patient should continue to use these medicines even if their condition improves.
After starting Pulveril, the patient should not stop taking their inhaled steroid (or steroid in tablets).
If the patient has hyperthyroidism, heart disease (including irregular or rapid heart rhythm), or diabetes (salmeterol may increase blood sugar levels), the doctor may monitor their condition more frequently. In patients with diabetes, the doctor may check their blood sugar levels more frequently than usual. It may also be necessary to modify the dose of antidiabetic medicines.
Pulveril and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, including asthma medicines and any inhalation medicines, as well as those that are available without a prescription. Using Pulveril with other medicines may not be appropriate.
Before using Pulveril, the patient should inform their doctor if they are currently being treated for a fungal infection with medicines containing ketokonazole or itraconazole, or if they are taking ritonavir for HIV treatment. These medicines may increase the risk of salmeterol side effects (including irregular heart rhythm) or worsen them.
While using Pulveril, the patient should avoid taking beta-adrenergic blockers, unless their doctor recommends otherwise. Beta-adrenergic blockers, including atenolol, propranolol, and sotalol, are most commonly used to treat high blood pressure or other heart diseases. The patient should inform their doctor or nurse if they are taking beta-adrenergic blockers or have been prescribed them recently, as they may weaken or cancel the effect of salmeterol.
Salmeterol may decrease blood potassium levels. In such cases, the patient may experience heart rhythm disturbances, muscle weakness, or cramps. This is more likely if the patient uses salmeterol with certain medicines used to treat high blood pressure (diuretics) and other medicines used to treat breathing disorders (such as theophylline or steroids). The doctor may occasionally order a blood potassium level test. Any doubts should be discussed with the doctor.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant, thinks they may be pregnant, or plans to become pregnant, they should inform their doctor or pharmacist before using Pulveril. They will assess whether it is possible to use Pulveril at that time.
Driving and using machines
Possible side effects of using Pulveril should not affect the patient's ability to drive or operate machines.
Pulveril contains ethanol
This medicine contains small amounts of ethanol (alcohol), less than 100 mg per dose.
3. HOW TO USE PULVERIL
This medicine should always be used as directed by the doctor or pharmacist. In case of doubts, the patient should consult their doctor or pharmacist.
Patients with asthma should always use Pulveril with an inhaled steroid.
Pulveril should be used daily until the doctor recommends stopping the treatment.
The patient may feel the onset of the medicine's effect on the first day of use.
Pulveril should be inhaled only through the mouth.
Dosage for adults and adolescents aged 12 years and older with asthma
The initial dose is usually 2 inhalations twice a day.
In patients with severe asthma, the doctor may increase the dose to 4 inhalations twice a day.
Children
Pulveril should not be used in children under 12 years of age, as its safety and efficacy have not been established in this age group.
Dosage for adults with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema
The initial dose is usually 2 inhalations twice a day.
This does not apply to children and adolescents.
Method of use
The doctor or pharmacist should demonstrate how to use the inhaler to the patient and check their technique from time to time. Incorrect use or use in a way other than described in this leaflet may result in the medicine not working effectively and not relieving asthma or COPD symptoms.
The medicine is contained in a pressurized container, placed in a plastic casing with a mouthpiece.
Checking the inhaler
Before first use, the patient should check if the inhaler is working properly.
- 1. Remove the cap from the mouthpiece by squeezing the sides gently with the thumb and index finger.
- 2. Shake the inhaler for at least 10 seconds. With the mouthpiece pointing away from the patient, press the canister twice to release two doses of the medicine into the air. If the inhaler has not been used for at least a week or has been cleaned, the patient should release the medicine once into the air.
Using the inhaler
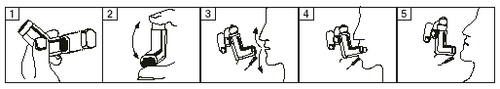
- 1. The patient should inhale while standing or sitting.
- 2. Remove the cap from the inhaler mouthpiece. Check that the inner and outer parts of the mouthpiece are clean (figure 1).
- 3. Before use, shake the inhaler well for at least 5 seconds (figure 2) to ensure that any foreign bodies are removed and the contents of the canister are evenly mixed.
- 4. Hold the inhaler vertically with the thumb on the base, below the mouthpiece.
- 5. Exhale as deeply as possible (figure 3).
- 6. Place the mouthpiece in the mouth between the teeth and wrap the lips around it without biting (figure 4).
- 7. Immediately after starting to inhale slowly (through the mouth), press the top of the inhaler to release a dose of the medicine and continue to inhale slowly and deeply (figure 4).
- 8. Hold the breath and remove the inhaler from the mouth. Hold the breath for as long as possible (figure 5).
- 9. If another inhalation is required, wait for about half a minute and then repeat the steps from 3 to 8.
- 10. After use, always replace the protective cap on the mouthpiece to protect it from dust and contamination. The cap should be pushed onto the mouthpiece and clicked into place.
The patient should practice the first few inhalations in front of a mirror. If they see a "mist" coming out of the inhaler or the corners of their mouth, they should start the inhalation again.
If the patient has difficulty using the inhaler, a spacer (such as Volumatic) may be helpful. They should consult their doctor or pharmacist. If a spacer is necessary, the patient should read the leaflet provided with it, which contains all the necessary information for proper use.
If the inhaler becomes very cold, the patient should remove the metal canister from the plastic casing and warm it in their hands for a few minutes. They should never use anything else for this purpose.
After warming the canister, the patient should release the medicine once into the air.
Cleaning the inhaler
To prevent the inhaler from clogging, it should be cleaned at least once a week.
To clean the inhaler, the patient should:
remove the mouthpiece cap;
not remove the metal canister from the plastic casing;
wipe the inside and outside of the mouthpiece and the plastic casing with a dry cloth or tissue;
release the medicine once into the air before the next use;
replace the mouthpiece cap.
Do not putthe metal canister in water.
Using a higher dose of Pulveril than recommended
It is important to use the inhaler as directed. If the patient accidentally uses a higher dose than recommended, they should consult their doctor or pharmacist. They may experience a faster heart rate, tremors, and (or) dizziness. Additionally, they may experience headache, muscle weakness, and joint pain.
Missing a dose of Pulveril
If the patient forgets to use their medicine, they should take the next dose at the usual time.
They should not take a double dose of the medicine to make up for the missed dose.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Pulveril can cause side effects, although not everybody gets them.
To minimize the risk of side effects, the doctor will prescribe the lowest dose that will control the patient's asthma or COPD symptoms. In patients using salmeterol, the following side effects have been reported.
Allergic reactions: the patient may experience sudden worsening of breathing after using salmeterol.
The following may occur:
wheezing or coughing;
rash, itching, swelling (usually of the face, lips, tongue, or throat).
If the patient experiences any of these symptoms or if they occur suddenly after using salmeterol, they should immediately inform their doctor. Allergic reactions to salmeterol are very rare (occurring in less than 1 in 10,000 patients).
Other possible side effects:
Common (may affect up to 1 in 10 people)
Muscle cramps.
Tremors, fast or irregular heart rhythm (palpitations), headache, tremors of the hands. Tremors occur more frequently if the patient uses more than 2 inhalations twice a day. These side effects do not last long, and their severity usually decreases during continued treatment with salmeterol.
Uncommon (may affect up to 1 in 100 people)
Rash.
Very fast heart rhythm (tachycardia), which occurs more frequently if the patient uses more than 2 inhalations twice a day.
Nervousness.
Rare (may affect up to 1 in 1,000 people)
Dizziness.
Difficulty sleeping (insomnia).
Decreased blood potassium levels (may manifest as irregular heart rhythm, muscle weakness, and (or) cramps). The doctor may occasionally order a blood potassium level test. In case of doubts, the patient should discuss them with their doctor or nurse.
Very rare (may affect up to 1 in 10,000 people)
Difficulty breathing or wheezing, which worsens immediately after using Pulveril. In such cases, the patient should stop using Pulveril.
The patient should use a rapidly acting inhaled bronchodilator to facilitate breathing and immediately inform their doctor.
Irrregular heart rhythm or extra heartbeats (arrhythmia). In such cases, the patient should not stop using Pulveril, but they should inform their doctor or nurse.
Increased blood sugar levels (hyperglycemia). In patients with diabetes, the doctor will check their blood sugar levels more frequently than usual. It may be necessary to adjust the dose of antidiabetic medicines.
Pain in the mouth or throat.
Nausea.
Pain, swelling of the joints.
Chest pain.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw; tel.: +48 22 49 21 301; fax: +48 22 49 21 309; website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. HOW TO STORE PULVERIL
Keep out of sight and reach of children.
After use, replace the protective cap on the mouthpiece and click it into place. Do not use force for this purpose.
Store in a temperature below 25°C.
Do not freeze.
The canister contains a pressurized liquid. Do not expose to temperatures above 50°C.
Do not pierce, crush, or burn the canister, even if it is empty.
Do not use Pulveril after the expiry date stated on the label and carton after EXP. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer use. This will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Pulveril contains
The active substance of the medicine is salmeterol (in the form of salmeterol xinafoate). Each dose delivered (from the inhaler) contains 25 micrograms of salmeterol (in the form of salmeterol xinafoate). This corresponds to a delivered dose (from the inhaler) containing 21 micrograms of salmeterol (in the form of salmeterol xinafoate).
The medicine also contains anhydrous ethanol, soy lecithin (E 322), and norflurane (HFA-134a).
What Pulveril looks like and contents of the pack
Inhaler with a non-freon propellant
A white suspension in an aluminum pressurized canister with a metering valve, with a green polypropylene mouthpiece and a light green polypropylene cap. Each canister contains 120 doses, and each dose contains 25 micrograms of salmeterol (in the form of salmeterol xinafoate).
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
Manufacturer/Importer:
Aeropharm GmbH
Francois-Mitterand-Allee 1
07407 Rudolstadt, Germany
Salutas Pharma GmbH
Otto-von-Guericke Allee 1
39179 Barleben, Germany
Fannin Limited
South County Business Park
Leopardstown
Dublin 18, Ireland
To obtain more detailed information about the medicine and its names in the Member States of the European Economic Area, the patient should contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
tel. +48 22 209 70 00
Date of last revision of the leaflet:08/2021
Sandoz logo
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAeropharm GmbH Fannin Limited Fannin (UK) Limited Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PulverilDosage form: Powder, 50 mcg/dose inh.Active substance: salmeterolManufacturer: Polfarmex S.A.Prescription requiredDosage form: Powder, 50 mcg/dose inh.Active substance: salmeterolPrescription requiredDosage form: Powder, 50 mcg/dose inh.Active substance: salmeterolPrescription required
Alternatives to Pulveril in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Pulveril in Ukraine
Alternative to Pulveril in Spain
Online doctors for Pulveril
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Pulveril – subject to medical assessment and local rules.














