
Ozased
Ask a doctor about a prescription for Ozased

How to use Ozased
Leaflet accompanying the packaging: information for the user
OZASED, 2 mg/ml, oral solution in a single-dose container
Midazolam
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- If the child experiences any side effects, including any side effects not listed in this leaflet, you should tell the doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is OZASED and what is it used for
- 2. Important information before using OZASED in children
- 3. How to use OZASED
- 4. Possible side effects
- 5. How to store OZASED
- 6. Contents of the packaging and other information
1. What is OZASED and what is it used for
OZASED contains midazolam. Midazolam belongs to a group of medicines called benzodiazepines.
OZASED is used in infants, children, and adolescents from 6 months to 17 years to induce
moderate sedation:
- before a surgical or diagnostic procedure to alleviate anxiety, agitation, and excitement associated with the procedure,
- as premedication before anesthesia.
2. Important information before using OZASED in children
When not to use OZASED:
- if the child is allergic (hypersensitive) to midazolam or any of the other ingredients of this medicine (listed in section 6),
- if the child has a neuromuscular disease that causes severe muscle weakness (myasthenia),
- if the child has significant breathing difficulties,
- if the child has a disease that causes frequent pauses in breathing during sleep (sleep apnea syndrome),
- if the child has severe liver disease.
Warnings and precautions
Before starting to use OZASED in children, you should discuss this with a doctor or pharmacist if:
- the child has a long-term illness (e.g., breathing or kidney, liver, or heart problems),
- the child is in poor general health,
- the child has had alcoholism or drug addiction,
- the child is under 6 months old.
OZASED and other medicines
You should tell the doctor or pharmacist about all medicines the child is taking or has recently taken, as well as any medicines the patient plans to take, especially if the child is taking any of the following medicines:
- medicines used to treat bacterial infections (antibiotics), e.g., erythromycin, clarithromycin, telithromycin, roxithromycin,
- medicines used to treat fungal infections (antifungal medicines), e.g., ketoconazole, voriconazole, fluconazole, itraconazole, and posaconazole,
- medicines used to treat stomach ulcers (anti-ulcer medicines), e.g., cimetidine and ranitidine,
- medicines used to treat epilepsy (antiepileptic medicines), e.g., phenytoin and carbamazepine,
- medicines used to treat high blood pressure (antihypertensive medicines), e.g., diltiazem and verapamil,
- medicines used to treat HIV and AIDS, e.g., saquinavir, including combination products containing ritonavir and efavirenz,
- a medicine used to prevent nausea and vomiting, e.g., aprepitant,
- a medicine used to reduce the amount of fat in the blood, e.g., atorvastatin,
- medicines used to treat depression that cause drowsiness (sedating antidepressants),
- other medicines used to treat depression (antidepressants), e.g., fluvoxamine,
- medicines used to treat cystic fibrosis, e.g., ivacaftor,
- medicines used to treat urinary incontinence, e.g., propiverine,
- medicines used to treat tuberculosis, e.g., rifampicin
- medicines used for anesthesia, e.g., inhalation anesthetics, propofol, ketamine, etomidate,
- medicines that cause drowsiness (sedatives),
- medicines used to treat severe pain (narcotic painkillers), e.g., fentanyl,
- medicines used to treat cough or opioid addiction (substitution therapy) containing opioids;
- medicines used to treat certain mental disorders, such as psychosis (antipsychotic medicines),
- medicines containing benzodiazepines used to treat anxiety disorders or sleep disorders (benzodiazepines used as anxiolytics or sedatives),
- medicines that relieve allergies (antihistamines),
- herbal medicines, e.g., St. John's wort, passionflower, turmeric.
OZASED with food, drink, and alcohol
Before administering sedatives, you should follow general guidelines for fasting.
The child should not drink alcohol while using OZASED. Alcohol may increase the sedative effect of this medicine and cause significant drowsiness.
The child should not drink grapefruit juice while using OZASED. Grapefruit juice may enhance the sedative effect of this medicine and cause significant drowsiness
Pregnancy and breastfeeding
Pregnancy
If the child is pregnant or the parent/caregiver suspects that the child is pregnant, they should ask the doctor for advice before giving this medicine.
Breastfeeding
If the child is a breastfeeding mother, she should be informed of the need to stop breastfeeding for 24 hours after administration of midazolam, as midazolam passes into breast milk in small amounts.
Driving and using machines
OZASED may cause drowsiness, forgetfulness, or affect concentration and coordination in the child. The child should not drive vehicles, ride a bicycle, or use tools or machines before the effects of the medicine have worn off completely. To get additional advice, you should contact the doctor.
OZASED contains sodium, ethanol, and gammadeks
The medicine contains less than 1 mmol (23 mg) of sodium per ampoule, i.e., the medicine is considered "sodium-free".
This medicine contains a maximum of 17.4 mg of alcohol (ethanol, orange flavoring) in each single dose, 5 ml ampoule, which is equivalent to 3.5 mg/ml (ethanol/solution) or 0.32% w/v.
The amount of ethanol in 1 ampoule of 5 ml of this medicine (17.4 mg) is equivalent to 0.2 ml of wine. The amount of ethanol in 2 ampoules of 5 ml of this medicine (34.8 mg) is equivalent to 0.4 ml of wine at the maximum dose of 20 mg of midazolam.
The small amount of alcohol in this medicine will not have noticeable effects.
This medicine contains 400 mg of gammadeks in each ampoule, which corresponds to 10 mg/kg body weight/day in the recommended dose and is below the acceptable daily exposure. Therefore, even if OZASED is accidentally administered at a dose of 0.5 mg/kg body weight, the amount of gammadeks taken will not exceed the acceptable daily exposure.
3. How to use OZASED
Instructions for use
OZASED should be administered orally.
OZASED will be administered to the child by medical personnel. The medicine will be administered in a place where equipment is available to monitor the child's condition and treat any side effects.
OZASED is not intended for self-administration.
After discharge from the hospital, the child should be accompanied by an adult. The patient may leave the operating room only after obtaining permission from the doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur during the use of midazolam. The frequency of their occurrence is unknown. The frequency cannot be determined based on currently available data.
Nervous system disorders:
- Prolonged/excessive sedation,
- Agitation, anxiety, hostility, anger, or aggression, excitement, confusion, euphoria (excessive feeling of happiness or excitement) or hallucinations (seeing and possibly hearing things that do not actually exist);
- Drowsiness,
- Dizziness,
- Difficulty with muscle coordination,
- Vertigo of labyrinthine origin,
- Speech disorders,
- Dry mouth,
- Excessive salivation,
- Urinary incontinence,
- Headache,
- Transient memory loss,
Immune system disorders,
- In sensitive individuals, hypersensitivity reactions and edema may occur,
Kounis syndrome, a serious allergic reaction characterized by chest pain, has been observed.
Cardiac disorders:
- Change in heart rate (slow or rapid heartbeat).
Respiratory disorders:
- Laryngospasm (laryngeal spasm causing difficulty breathing and loud breathing), breathing difficulties (slow breathing), wheezing,
- Loud breathing,
- Hiccup,
Gastrointestinal disorders:
- Vomiting,
- Nausea
Eyes disorders:
- Blurred vision,
- Double vision,
Skin disorders:
- Itching, skin rash with red, raised, itchy blisters (hives),
- Skin rash.
General disorders and administration site conditions:
- Unusual fatigue,
- Feeling of weakness.
Reporting side effects
If the child experiences any side effects, including any side effects not listed in the leaflet, you should tell the doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store OZASED
The medicine should be stored out of sight and reach of children.
Do not use the medicine after the expiry date stated on the ampoule label, blister, or carton after "EXP". The expiry date refers to the last day of the month.
Store in the original packaging to protect from light. Do not store above 25°C. Do not store in the refrigerator or freeze.
6. Contents of the packaging and other information
What OZASED contains
- The active substance is midazolam.
- The other ingredients are: citric acid monohydrate, gammadeks, sucralose, orange flavor (containing in particular 70-80% ethanol), sodium hydroxide (for pH adjustment), water for injections
What OZASED looks like and contents of the pack
The packaging of OZASED contains one 5 ml glass ampoule of type I orange glass, one tube with a filter, and one oral applicator packed together in a single blister.
OZASED is available in 3 different pack sizes:
- packaging with one blister
- packaging with 5 blisters
- packaging with 10 blisters
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Istituto Gentili S.r.l.
Via San Giuseppe Cottolengo 15
20143 Milan
Italy
[email protected]
Manufacturer
Valdepharm
Parc Industriel d’Incarville – CS 10606
27106 Val-De-Reuil Cedex
France
This medicinal product is authorized in the Member States of the European
Economic Area under the following names:
Austria
OZASED 2 mg/ml Lösung zum Einnehmen im Einzeldosisbehältnis
Belgium
Ozalin 2 mg/ml solution buvable en récipient unidose /
drank in verpakking voor éénmalig gebruik /
Lösung zum Einnehmen im Einzeldosisbehältnis
Denmark
Ozalin 2 mg/ml oral opløsning i enkeltdosisbeholder
Finland
Ozalin 2 mg/ml oraaliliuos kerta-annospakkaus
France
Ozalin 2 mg/ml solution buvable en récipient unidose
Germany
Ozalin 2 mg/ml Lösung zum Einnehmen im Einzeldosisbehältnis
Greece
Ozalin 2 mg/ml πόσιμο διάλυμα σε περιέκτη μίας δόσης
Ireland
Ozalin 2 mg/ml oral solution in single-dose container
Italy
Ozased 2 mg/ml soluzione orale in contenitore monodose
Norway
Ozalin 2 mg/ml mikstur, oppløsning i endosebeholder
Poland
OZASED, 2 mg/ml, roztwór doustny w pojemniku jednodawkowym
Portugal
Ozalin 2 mg/ml solução oral em recipiente unidose
Spain
Ozalin 2 mg/ml solución oral en envase unidosis
Netherlands
Ozalin 2 mg/ml drank in verpakking voor eenmalig gebruik
Date of last revision of the leaflet: ------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
The solution should be inspected visually before use. Do not use this medicine if you notice any visible signs of solution degradation or packaging damage. OZASED should be administered only with the dedicated, special oral applicator with a scale in kg:
How to open the ampoule
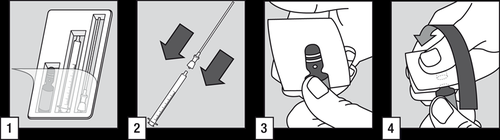
- (1) Administration of the medicinal product to the patient requires the use of an ampoule, tube with a filter, and oral applicator.
- (2) Put the tube with a filter on the end of the oral applicator.
- (3) Tap the top of the ampoule to make the liquid flow down. Cover the top of the ampoule with a cloth and hold it, placing your thumb on the white dot.
- (4) Hold the ampoule firmly with the white dot facing upwards and towards you. Press the top of the ampoule away from you, which will break off easily.
Preparing and administering the solution
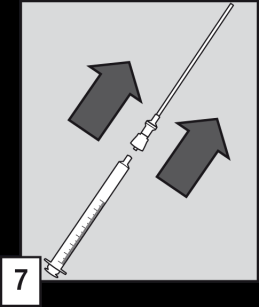
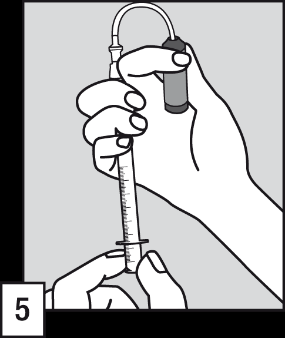
- (5) Put the tube with a filter into the ampoule. Before drawing up the dose and to eliminate any air from the tube with a filter, it is recommended to briefly pump the oral applicator (filling and emptying) the solution inside the ampoule.
- (6) Holding the ampoule vertically, fill the oral applicator to the mark on the scale corresponding to the patient's body weight in kilograms (kg). Align the line mark with the top of the collar to draw up the correct dose.
- (7) Remove the tube with a filter from the end of the oral applicator.
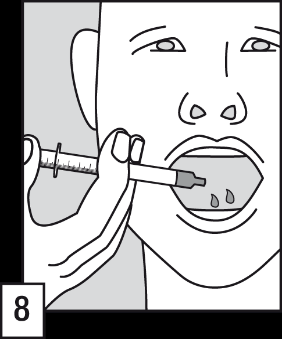
- (8) Empty the contents of the oral applicator into the patient's mouth. The solution should be swallowed immediately.
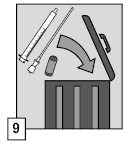
- (9) After use, dispose of the ampoule, tube with a filter, oral applicator, and any unused remnants of the medicinal product into a container intended for this purpose, in accordance with local regulations regarding controlled substances and pharmaceutical accessories.
Dosage
The dose should be adjusted according to the patient's body weight.
OZASED should be used orally in a single dose of 0.25 mg/kg body weight in children from 6 months.
The maximum dose should not exceed 20 mg of midazolam (which corresponds to 2 ampoules) even in children and adolescents with a body weight over 80 kg.
In the case of obese children and adolescents, the dose should be administered according to the actual body weight and not exceed 20 mg.
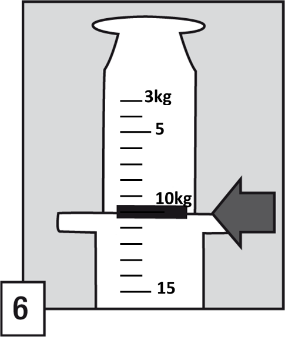
The oral applicator has a scale in kilograms, from 3 kg to 40 kg body weight , with three types of scale marks:
- A small scale mark corresponds to 1 kg, i.e.: 0.25 mg of midazolam,
- A medium scale mark corresponds to 5 kg, i.e.: 1.25 mg of midazolam,
- A large scale mark corresponds to 10 kg, i.e.: 2.50 mg of midazolam
In the case of patients with a body weight over 40 kg, two ampoules are required. The minimum dose to be drawn from the ampoule should correspond to the dose for 3 kg. In the case of patients with a body weight of 41 and 42 kg, who require the use of more than one ampoule, the dose should be drawn in a smaller amount than for 40 kg from the first ampoule and supplemented to the dose in the second ampoule, see examples below:
- In the case of a patient with a body weight of 41 kg, it is recommended to take the dose for 30 kg from the first ampoule and the dose for 11 kg from the second ampoule
- In the case of a patient with a body weight of 42 kg, it is recommended to take the dose for 30 kg from the first ampoule and the dose for 12 kg from the second ampoule.
The oral applicator and tube with a filter are single-use devices for drawing up and administering the dose.
OZASED should be administered approximately 30 minutes before the procedure or anesthesia.
OZASED is not recommended for use in newborns (premature and full-term) or infants under 6 months.
In case of overdose, if the patient is conscious, vomiting should be induced as soon as possible (within an hour of administration of midazolam), or gastric lavage should be performed with protection of the airways, if the patient is unconscious. If gastric lavage is not effective, activated charcoal should be administered to reduce absorption.
Flumazenil, a benzodiazepine antagonist, is indicated in case of severe poisoning, accompanied by respiratory depression or coma. This treatment can only be performed under close supervision and in accordance with local guidelines.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterValdepharm
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OzasedDosage form: Tablets, 15 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Tablets, 7.5 mgActive substance: midazolamManufacturer: Recipharm Leganes S.L.U. Roche Polska Sp. z o.o.Prescription requiredDosage form: Solution, 10 mgActive substance: midazolamManufacturer: MoNo chem-pharm. Produkte GmbHPrescription required
Alternatives to Ozased in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ozased in Spain
Alternative to Ozased in Ukraine
Online doctors for Ozased
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ozased – subject to medical assessment and local rules.









