
Otrivin Regeneracia

Ask a doctor about a prescription for Otrivin Regeneracia

How to use Otrivin Regeneracia
Package Leaflet: Information for the Patient
Otrivin Regeneracja, (1 mg + 50 mg)/ml, Nasal Spray, Solution
Xylometazoline Hydrochloride + Dexpanthenol
Read the Leaflet Carefully Before Using the Medication, as it Contains Important Information for the Patient.
This Medication Should Always be Used Exactly as Described in the Patient Leaflet or as Directed by a Doctor or Pharmacist.
- Keep this Leaflet, You May Need to Read it Again.
- If You Have any Further Questions, Ask Your Doctor or Pharmacist.
- If You Experience any Side Effects, Including those not Listed in this Leaflet, Tell Your Doctor or Pharmacist. See Section 4.
- If There is no Improvement After 7 Days or if You Feel Worse, Contact a Doctor.
Table of Contents of the Leaflet:
- 1. What is Otrivin Regeneracja and What is it Used for
- 2. Important Information Before Using Otrivin Regeneracja
- 3. How to Use Otrivin Regeneracja
- 4. Possible Side Effects
- 5. How to Store Otrivin Regeneracja
- 6. Contents of the Pack and Other Information
1. What is Otrivin Regeneracja and What is it Used for
Otrivin Regeneracja is a Nasal Spray.
Otrivin Regeneracja Contains the Active Substance Xylometazoline Hydrochloride, which has a Vasoconstrictive Effect and Reduces Swelling of the Nasal Mucosa.
Otrivin Regeneracja also Contains the Active Substance Dexpanthenol, which is a Derivative of Pantothenic Acid - a Vitamin that is Necessary for all Human Tissues and is Found in the Daily Diet.
Otrivin Regeneracja is Intended for Use in Adults and Adolescents from 12 Years of Age:
- to Reduce Swelling of the Nasal Mucosa in Rhinitis, as well as to Support Wound Healing and Protect the Nasal Mucosa, to Relieve Vasomotor Rhinitis and to Treat Impaired Nasal Breathing After Nasal Surgery,
- to Reduce Swelling of the Nasal Mucosa in Rhinitis Associated with Acute Sinusitis (Rhinitis and Sinusitis).
2. Important Information Before Using Otrivin Regeneracja
When Not to Use Otrivin Regeneracja
- if the Patient is Hypersensitive to Xylometazoline Hydrochloride or Dexpanthenol, or to any of the other Ingredients of this Medication (Listed in Section 6),
- if the Patient has Dry Rhinitis, Including with the Formation of Crusts (Dry or Atrophic Rhinitis),
- if the Patient has Undergone Nasal Surgery, e.g. Removal of the Pituitary Gland or any other Surgery Involving Exposure of the Meninges,
- if the Patient has Increased Eye Pressure, Especially Narrow-Angle Glaucoma,
- in Infants and Children Under 12 Years of Age.
Warnings and Precautions
Before Starting to Use Otrivin Regeneracja, Discuss it with a Doctor or Pharmacist:
- if the Patient is Being Treated with Monoamine Oxidase Inhibitors (MAOIs) or has Received these Medications in the Last Two Weeks, as well as if they have Received other Medications that may Cause an Increase in Blood Pressure,
- in Patients Using Tricyclic and Tetracyclic Antidepressants,
- if the Patient has Severe Cardiovascular Disease (e.g. Coronary Artery Disease, Long QT Syndrome) or High Blood Pressure (Hypertension),
- if the Patient has a Pheochromocytoma (Adrenal Gland Tumor),
- if the Patient has Metabolic Disorders, such as Hyperthyroidism or Diabetes,
- if the Patient has a Metabolic Disease (Porphyria),
- if the Patient has Prostatic Hyperplasia (Prostate Enlargement).
In Order to Avoid the Spread of Infection, one Package of the Medication Should be Used by only one Patient, and the Nozzle Should be Rinsed After Use.
In Very Rare Cases, Xylometazoline may Cause Insomnia. In this Case, it is Recommended to Avoid Using the Medication in the Late Evening or at Night.
Elderly Patients
Elderly Patients may be more Susceptible to the Side Effects of this Medication.
During Prolonged Use of the Medication, it Should be Used only Under Medical Supervision Due to the Risk of Thinning of the Nasal Mucosa.
Children and Adolescents
Long-Term Use of the Medication (More than 10 Consecutive Days) and its Use in Higher Doses than Recommended Should be Avoided, Especially in Children, as this may Cause Hyperemia of the Nasal Mucosa and Consequently Narrowing of the Airways.
Continued Use of the Medication may Lead to Secondary Medication-Induced Edema and Atrophy of the Nasal Mucosa.
The Concentration of Active Substances in Otrivin Regeneracja has been Chosen for Use in Adults and Adolescents from 12 Years of Age, and therefore the Medication Should not be Used in Infants or Children Under 12 Years of Age.
For Children from 1 Year of Age to 11 Years, there are Available other Medications in the Form of a Nasal Spray, Containing a Lower Dose of the Active Substance that Reduces Swelling of the Nasal Mucosa - Xylometazoline Hydrochloride.
Otrivin Regeneracja and Other Medications
Tell Your Doctor or Pharmacist About all Medications You are Currently Taking, or Have Recently Taken, as well as any Medications You Plan to Take.
Using Otrivin Regeneracja Together with Medications that Improve Mood (Monoamine Oxidase Inhibitors or Tricyclic and Tetracyclic Antidepressants), as well as other Medications that Increase Blood Pressure, may Cause an Increase in Blood Pressure as a Result of the Effect of these Substances on the Cardiovascular System.
Pregnancy, Breastfeeding, and Fertility
Pregnancy
There is no Sufficient Data on the Use of Xylometazoline Hydrochloride and Dexpanthenol in Pregnant Women, therefore Otrivin Regeneracja Should not be Used in Pregnant Women.
Breastfeeding
It is not Known whether Xylometazoline and Dexpanthenol Pass into Breast Milk, therefore Otrivin Regeneracja Should not be Used in Breastfeeding Women.
Fertility
There is no Sufficient Data on the Effect of Xylometazoline Hydrochloride and Dexpanthenol on Fertility in Humans.
If You are Pregnant, Breastfeeding, or Think You may be Pregnant, or are Planning to Have a Child, Consult a Doctor or Pharmacist Before Using this Medication.
Driving and Using Machines
Side Effects Should not Occur when the Medication is Used in the Recommended Doses.
However, if You Feel Drowsy or Sleepy, Do not Drive or Operate Machinery.
Otrivin Regeneracja Contains Benzalkonium Chloride
This Medication Contains 0.02 mg of Benzalkonium Chloride in Each Dose of the Spray, which Corresponds to 0.2 mg of Benzalkonium Chloride in Each 1 ml of the Solution.
This Substance may Cause Irritation or Swelling of the Nasal Mucosa, Especially if Used for a Long Time.
If You Experience any Discomfort While Using Otrivin Regeneracja, Inform a Doctor or Pharmacist.
3. How to Use Otrivin Regeneracja
This Medication Should Always be Used Exactly as Described in the Patient Leaflet.
In Case of Doubt, Consult a Doctor or Pharmacist.
Method of Administration
Otrivin Regeneracja is Intended for Nasal Use.
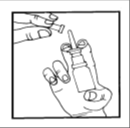
- 1. Remove the Protective Cap from the Nozzle of the Applicator.
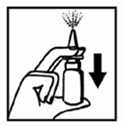
- 2. Before the First Administration, Press the Pump Several Times Until the Medication is Released into the Air.
There is no Need to Repeat this Procedure for Subsequent Administrations of the Medication.
The Pump will be Ready for Further Use Throughout the Recommended Treatment Period.
If, However, After Pressing the Pump, the Medication is not Released or if the Product is not Used for Several Days, it is Necessary to Prepare the Medication Again by Pressing the Pump Twice.
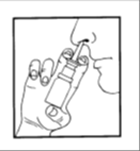
Be Careful not to Spray the Medication into the Eyes.
- 3. Clean the Nose.
- 4. Hold the Bottle Upright, with the Thumb on the Bottom of the Bottle and the Nozzle Between Two Fingers.
- 5. Gently Tilt Your Head Forward and Place the Nozzle in the Nostril.
- 6. Press the Pump and at the Same Time Gently Inhale Through the Nose. Repeat the Procedure when Administering the Medication to the Other Nostril.
- 7. Immediately After Use, Wipe the Nozzle of the Applicator and Put on the Protective Cap.
- 8. In Order to Avoid the Spread of Infection, the Package of the Spray Should be Used by only one Person.
Dosage
In Adults and Adolescents, Unless Otherwise Directed by a Doctor, Use one Dose of Otrivin Regeneracja Spray into Each Nostril, up to 3 Times a Day, as Needed.
The Dosage Depends on Individual Sensitivity and Clinical Response. Do not Use more than 3 Times a Day into Each Nostril.
Duration of Treatment
Otrivin Regeneracja Should not be Used for more than 7 Days, Unless a Doctor has Specifically Recommended Longer Use.
Prolonged Use of this Medication may Lead to Chronic Swelling, and Ultimately to Loss of the Nasal Mucosa.
Use in Children
Otrivin Regeneracja Should not be Used in Children Under 12 Years of Age. See Section 2 "When not to Use Otrivin Regeneracja".
Use of a Higher than Recommended Dose of Otrivin Regeneracja
In Case of Overdose or Accidental Ingestion, the Following Symptoms may Occur:
Narrowing of the Pupils, Dilation of the Pupils, Fever, Sweating, Pallor, Cyanosis, Nausea, Cramps, Cardiovascular Disorders, such as Heart Rhythm Disorders (Tachycardia, Bradycardia, other Arrhythmias), Circulatory Collapse, Cardiac Arrest, High Blood Pressure (Hypertension), Pulmonary Disorders (Pulmonary Edema, Difficulty Breathing), Mental Disorders.
Additionally, Drowsiness, Decreased Body Temperature, Decreased Heart Rate, Hypotension Resembling Shock, Respiratory Failure, and Loss of Consciousness (Coma) may Occur.
In Case of Suspected Overdose, Immediately Contact a Doctor. Optionally, Appropriate Measures may be Taken.
Missed Dose of Otrivin Regeneracja
Do not Use a Double Dose to Make up for a Missed Dose. Continue Treatment as Recommended.
If You Have any Further Questions About Using this Medication, Consult a Doctor or Pharmacist.
4. Possible Side Effects
Like all Medications, Otrivin Regeneracja can Cause Side Effects, although not Everybody gets them.
Uncommon Side Effects (Occurring in no more than 1 in 100 People)
- Allergic Reactions (Rash, Itching, Swelling of the Skin and Mucous Membranes)
- Nosebleeds
Rare Side Effects (Occurring in no more than 1 in 1000 People)
- Palpitations
- Accelerated Heart Rate (Tachycardia)
- Increased Blood Pressure (Hypertension)
Very Rare Side Effects (Occurring in no more than 1 in 10,000 People)
- Agitation
- Insomnia
- Fatigue (Drowsiness, Sedation)
- Headaches
- Hallucinations (Especially in Children)
- Irregular Heartbeat (Heart Rhythm Disorders)
- Increased Swelling of the Nasal Mucosa due to Decreased Effect of the Medication
- Cramps (Especially in Children)
Frequency Not Known (Frequency Cannot be Estimated from the Available Data)
- Burning or Dryness of the Nasal Mucosa
- Sneezing
Reporting Side Effects
If You Experience any Side Effects, Including those not Listed in this Leaflet, Tell Your Doctor or Pharmacist.
Side Effects can be Reported Directly to the Department for Monitoring of Adverse Reactions of Medicinal Products, Medical Devices and Biocidal Products of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl.
Side Effects can also be Reported to the Marketing Authorization Holder.
Reporting Side Effects will Help to Gather more Information on the Safety of the Medication.
5. How to Store Otrivin Regeneracja
Keep the Medication out of the Sight and Reach of Children.
Do not Use this Medication After the Expiry Date Stated on the Packaging. The Expiry Date Refers to the Last Day of the Month Stated.
The Expiry Date Applies even if the Packaging has been Opened.
Do not Store Above 25°C.
Medications Should not be Disposed of via Wastewater or Household Waste. Ask Your Pharmacist How to Dispose of Medications that are no Longer Needed. This will Help to Protect the Environment.
6. Contents of the Pack and Other Information
What Otrivin Regeneracja Contains
- The Active Substances of the Medication are Xylometazoline Hydrochloride and Dexpanthenol. One 0.1 ml Dose of the Spray Solution Contains 0.1 mg of Xylometazoline Hydrochloride and 5.0 mg of Dexpanthenol.
- The Other Ingredients of the Medication are: Benzalkonium Chloride (Solution), Potassium Dihydrogen Phosphate, Disodium Phosphate Dodecahydrate, Purified Water.
What Otrivin Regeneracja Looks Like and What the Pack Contains
Otrivin Regeneracja is a Colorless, Transparent Solution.
The Medication Packaging is a Plastic Bottle with a Capacity of 15 ml with a Dosage Device and a Nasal Applicator with a Protective Cap, in a Box.
Each Bottle Contains 10 ml of the Solution.
Marketing Authorization Holder
Haleon Poland Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warsaw
Tel: 800 702 849
Manufacturer
Zakłady Farmaceutyczne Polpharma S.A.
Oddział Medana w Sieradzu
ul. Władysława Łokietka 10
98-200 Sieradz, Poland
Haleon Germany GmbH
Barthstraße 4
80339 Munich
Germany
This Medication has been Authorized for Marketing in the Member States of the European Union Under the Following Trade Names:
Austria
Otrivin plus Dexpanthenol 1 mg/ml + 50 mg/ml Nasenspray, Lösung
Bulgaria
Отривин Плюс 1 mg/ml + 50 mg/ml спрей за нос, разтвор
Estonia
Otrinazil
Germany
Otriven Protect 1 mg/ml + 50 mg/ml Nasenspray, Lösung
Greece
Xylometazoline + Dexpanthenol / GSK 1mg/ml+50mg/ml ρινικό εκνέφωμα, διάλυμα
Hungary
Otrivin Extra 1 mg/ml + 50 mg/ml adagoló oldatos orrspray
Latvia
Otrinazil 1 mg/50 mg/ml deguna aerosols, šķīdums
Lithuania
Otrinazil 1 mg/50 mg/ml nosies purškalas (tirpalas)
Poland
Otrivin Regeneracja
Portugal
Vibrocil ActilongProtect
Romania
Vibrocil Actilong Plus 1mg/ml + 50mg/ml spray nazal soluţie
Slovakia
Otrivin Plus 1 mg/ml + 50 mg/ml
Spain
Rhinovín Cuidado Activo 1mg/ml + 50mg/ml Solución para pulverización nasal
Date of Last Revision of the Leaflet: July 2024
Other Sources of Information
Detailed Information About this Medication is Available on the Website of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products http://www.urpl.gov.pl/pl
Additional Information for the User
The Function of a Healthy Nose is to Warm, Humidify and Filter the Inhaled Air.
This Protects the Bronchi from Cold Air from the Outside and Prevents the Nose from Drying Out.
The Inside of the Nose is Lined with a Well-Vascularized Mucous Membrane.
The Mucous Membrane can be Damaged by Various Factors, such as a Cold or Surgical Procedures.
This Leads to Swelling of the Nasal Mucosa, which in Turn Makes it Difficult to Breathe Through the Nose and Causes a Feeling of a "Stuffy Nose".
The Otrivin Regeneracja Nasal Spray Containing Xylometazoline Hydrochloride and Dexpanthenol as Active Substances is Used Directly on the Nasal Mucosa to Counteract Swelling Caused by a Cold or Surgical Procedure in the Nose.
Xylometazoline Hydrochloride Causes a Long-Lasting Reduction in Swelling of the Nasal Mucosa Within a Few Minutes of Administration, Making it Easier to Breathe Through the Nose.
This Effect is Further Enhanced by the Combination with Dexpanthenol.
Dexpanthenol is a Derivative of Pantothenic Acid - a Vitamin that is Necessary for all Human Tissues and is Found in the Daily Diet.
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterHaleon Germany GmbH Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Medana w Sieradzu
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Otrivin RegeneraciaDosage form: Aerosol, (0.05 mg + 5 mg)/doseActive substance: xylometazolineManufacturer: Klosterfrau Berlin GmbHPrescription not requiredDosage form: Aerosol, (0.1 mg + 5 mg)/doseActive substance: xylometazolineManufacturer: Klosterfrau Berlin GmbHPrescription not requiredDosage form: Aerosol, (0.5 mg + 0.6 mg)/mlActive substance: xylometazolinePrescription not required
Alternatives to Otrivin Regeneracia in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Otrivin Regeneracia in Ukraina
Alternative to Otrivin Regeneracia in Hiszpania
Online doctors for Otrivin Regeneracia
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Otrivin Regeneracia – subject to medical assessment and local rules.








