
Ontipria

Ask a doctor about a prescription for Ontipria

How to use Ontipria
Package Leaflet: Information for the User
Ontipria, 18 micrograms, inhalation powder in a hard capsule
Tiotropium
Read the package leaflet carefully before using the medicine, as it contains
important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Contents of the package leaflet
- 1. What Ontipria is and what it is used for
- 2. Important information before using Ontipria
- 3. How to use Ontipria
- 4. Possible side effects
- 5. How to store Ontipria
- 6. Contents of the pack and other information
1. What Ontipria is and what it is used for
Ontipria, 18 micrograms, inhalation powder in a hard capsule makes it easier for people with chronic obstructive pulmonary disease (COPD) to breathe. COPD is a long-term lung disease that causes shortness of breath and coughing. The name COPD is associated with chronic bronchitis and emphysema. COPD is a chronic disease, so Ontipria, 18 micrograms, inhalation powder in a hard capsule should be taken every day, not just when breathing problems or other COPD symptoms occur.
Ontipria, 18 micrograms, is a long-acting bronchodilator that helps to widen the airways and make it easier for air to get in and out of the lungs. Regular use of Ontipria, 18 micrograms, may also help reduce persistent shortness of breath associated with the disease and help reduce the impact of the disease on daily life.
The medicine also helps to maintain activity for longer. Daily use of Ontipria will also help prevent sudden, short-term worsening of COPD symptoms, which can last for several days.
The effect of the medicine lasts for 24 hours, so it should only be used once a day.
For proper dosing of Ontipria, 18 micrograms, see section 3.
Instructions on how to use Ontipria, 18 micrograms, and instructions for use are available on another page of this leaflet.
2. Important information before using Ontipria
When not to use Ontipria
- if you are allergic to tiotropium or any of the other ingredients of this medicine (listed in section 6),
- if you are allergic to atropine or its derivatives, such as ipratropium or oxytropium.
Warnings and precautions
Before starting to use Ontipria, discuss it with your doctor or pharmacist.
- You should consult your doctor if you have narrow-angle glaucoma, prostate problems, or difficulty urinating.
- You should consult your doctor if you have kidney problems.
- This medicine is intended for maintenance therapy in patients with chronic obstructive pulmonary disease. It should not be used to treat sudden attacks of shortness of breath or wheezing.
- After using the medicine, immediate allergic reactions, such as rash, swelling, itching, wheezing, or shortness of breath, may occur. In this case, you should contact your doctor immediately.
- As with other inhaled medicines, after using Ontipria, 18 micrograms, you may experience a feeling of pressure in the chest, coughing, wheezing, or shortness of breath immediately after inhaling the medicine. In this case, you should contact your doctor immediately.
- You should not allow the powder to get into your eyes during inhalation, as it may cause the occurrence or worsening of symptoms of narrow-angle glaucoma, which is an eye disease. Eye pain or discomfort, blurred vision, seeing a rainbow-colored halo around a light source, or changed color vision, along with redness of the eyes, may be a sign of acute narrow-angle glaucoma. Headache, nausea, and vomiting may accompany eye disorders. If symptoms of narrow-angle glaucoma occur, you should stop using tiotropium bromide and contact your doctor immediately, preferably an ophthalmologist.
- Dryness of the oral mucosa occurring during the use of the medicine, related to its anticholinergic effect, may cause tooth decay after a longer period, so you should remember to maintain oral hygiene.
- If you have had a heart attack in the last 6 months, or if you have had unstable or life-threatening heart rhythm or severe heart failure in the last year, you should inform your doctor. It is important to make the right decision about whether Ontipria can be used in your case.
- Ontipria, 18 micrograms, should not be used more than once a day.
Children and adolescents
Ontipria is not recommended for children and adolescents under 18 years of age.
Ontipria and other medicines
Tell your doctor or pharmacist about all medicines (including those available without a prescription) that you are taking now or have taken recently, as well as any medicines that you plan to take.
Tell your doctor or pharmacist about other medicines used to treat lung disease, such as ipratropium or oxytropium.
No interactions have been reported when using Ontipria, 18 micrograms, with other medicines used to treat COPD, such as inhaled rescue medicines, e.g., salbutamol, methylxanthines, e.g., theophylline, and/or oral and inhaled steroids, e.g., prednisolone.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before using this medicine. You should not use this medicine unless your doctor has told you to.
Driving and using machines
If you experience dizziness, blurred vision, or headache, do not drive or operate machinery. You should not drive if you experience any of these symptoms.
Ontipria contains lactose
If you have been told that you have an intolerance to some sugars, you should contact your doctor before taking this medicine.
Lactose contains small amounts of proteins that can cause allergic symptoms.
3. How to use Ontipria
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Inhale the contents of one capsule (18 micrograms of tiotropium) once daily. Do not use more than the recommended dose.
Take the capsule at the same time each day. This is important because Ontipria, 18 micrograms, works for 24 hours.
The capsules are for inhalation use only and should not be swallowed.
Do not swallow the capsules.
The MRX003-T10 DPI dry powder inhaler (hereinafter referred to as the "inhaler"), into which the Ontipria capsule is inserted, pierces the capsule, allowing the powder to be inhaled.
You should make sure you have an inhaler and use it correctly. The instructions for using the inhaler are on another page of this leaflet.
Make sure you do not breathe out into the inhaler.
If you have any difficulties with using the inhaler, you should contact your doctor, nurse, or pharmacist to show you how to use it correctly.
The inhaler should be cleaned once a month. The instructions for cleaning the inhaler are in the rest of this leaflet.
Do not allow Ontipria, 18 micrograms, to get into your eyes. This may cause blurred vision, eye pain, and/or redness of the eyes. Rinse your eyes immediately with warm water and contact your doctor.
If you feel that your breathing difficulties are worsening, you should contact your doctor immediately.
Using a higher dose of Ontipria than recommended
If you use more than 1 capsule of the medicine per day, you should contact your doctor immediately. You may be at increased risk of side effects, such as dryness of the oral mucosa, constipation, difficulty urinating, rapid heartbeat, or blurred vision.
Missing a dose of Ontipria
If you forget to take a dose, take it as soon as you remember. Never take two doses at the same time or on the same day. Take the next dose at the usual time.
Stopping use of Ontipria
Before stopping use of Ontipria, 18 micrograms, you should talk to your doctor or pharmacist.
After stopping use of Ontipria, your COPD symptoms may worsen.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported by patients using the medicine.
Side effects are listed by frequency: common, uncommon, rare, or frequency not known.
Common (may affect up to 1 in 10 people):
- dryness of the oral mucosa (usually mild)
Uncommon (may affect up to 1 in 100 people):
- dizziness
- headache
- taste disturbances
- blurred vision
- irregular heartbeat (atrial fibrillation)
- pharyngitis
- hoarseness (dysphonia)
- cough
- heartburn (gastroesophageal reflux disease)
- constipation
- fungal infections of the mouth and throat (oral thrush)
- rash
- difficulty urinating (urinary retention)
- painful urination
Rare (may affect up to 1 in 1,000 people):
- sleep disorders (insomnia)
- seeing a rainbow-colored halo around a light source or changed color vision, along with redness of the eyes (glaucoma)
- increased eye pressure
- irregular heartbeat (supraventricular tachycardia)
- rapid heartbeat (tachycardia)
- feeling of rapid heartbeat (palpitations)
- chest pressure associated with coughing, wheezing, or shortness of breath occurring immediately after inhaling the medicine (bronchospasm)
- nasal bleeding
- laryngitis
- sinusitis
- intestinal obstruction or lack of bowel movements (intestinal obstruction, including paralytic ileus)
- gingivitis
- glossitis
- difficulty swallowing (dysphagia)
- stomatitis
- nausea
- hypersensitivity, including immediate reactions
- severe allergic reaction, which can cause swelling in the face or throat (angioedema)
- hives
- itching
- urinary tract infection
Frequency not known (frequency cannot be estimated from the available data):
- dehydration
- tooth decay
- severe allergic reactions (anaphylactic reactions)
- infections or ulcers of the skin
- dry skin
- joint swelling
Severe side effects, such as allergic reactions that can cause swelling in the face or throat (angioedema), or other hypersensitivity reactions (such as a sudden drop in blood pressure or dizziness) may occur alone or as part of a severe allergic reaction (anaphylactic reaction) after using Ontipria, 18 micrograms. Additionally, as with other inhaled medicines, some patients may experience unexpected chest pressure, coughing, wheezing, or shortness of breath immediately after inhaling the medicine (bronchospasm). If you experience any of these symptoms, you should contact your doctor immediately.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
5. How to store Ontipria
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the blister and carton after "EXP". The expiry date refers to the last day of that month.
The inhaler should be discarded no later than 6 months after first use.
Use the capsule immediately after opening the blister.
After removing the first capsule from the blister, use the capsules from the same blister for the next 9 days, one capsule per day.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Ontipria contains
The active substance is tiotropium.
Each capsule contains 18 micrograms of tiotropium (as tiotropium bromide). When inhaled from the inhaler, 10 micrograms of tiotropium are delivered.
The other ingredients are lactose monohydrate (capsule contents), hypromellose (type 2906, 4.5 mPa), (capsule shell), ammonium hydroxide, butyl alcohol, anhydrous ethanol, iron oxide (E 172), isopropyl alcohol, potassium hydroxide, propylene glycol, shellac, purified water (ink).
What Ontipria looks like and contents of the pack
Ontipria, inhalation powder, is a colorless, transparent hard capsule, size 3, with "T10" printed on the capsule, containing a white powder.
The medicine is packaged in perforated blisters, each containing 10 capsules. The carton contains blisters and an inhaler. The inhaler is white with a red button.
Available pack sizes:
Carton containing 30 capsules (3 blisters) and one MRX003-T10 dry powder inhaler.
Carton containing 60 capsules (6 blisters) and one MRX003-T10 dry powder inhaler.
Carton containing 90 capsules (9 blisters) and one MRX003-T10 dry powder inhaler.
Not all pack sizes may be marketed.
Marketing authorization holder
Zentiva, k.s.
U kabelovny 130
Dolní Měcholupy
102 37 Prague 10
Czech Republic
Manufacturer
Helm Pharmaceuticals GmbH
Nordkanalstrasse 28
20097 Hamburg
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Bulgaria, Portugal, Slovakia: Plubrom
Czech Republic, Romania, Slovenia: Dilochob
Estonia, Latvia, Lithuania, Hungary, Poland: Ontipria
Croatia: Plubir
For more information about this medicine, please contact the representative of the marketing authorization holder in Poland:
Zentiva Polska Sp. z o.o.
ul. Bonifraterska 17
00-203 Warsaw
tel.: +48 22 375 92 00
Date of last revision of the leaflet:October 2024
Instructions for use and maintenance of the MRX003-T10 inhaler
The MRX003-T10 dry powder inhaler allows you to inhale the medicine contained in the Ontipria capsules prescribed by your doctor for breathing disorders.
Remember to strictly follow your doctor's instructions for using Ontipria. The MRX003-T10 DPI inhaler is designed for Ontipria capsules, and patients should not use it to administer other medicines.
Inhalations with capsules can only be performed using the MRX003-T10 DPI inhaler.
Do not use other inhalers to administer Ontipria capsules. Each capsule contains a small amount of powder. Do not open the capsule, as it may not be suitable for use.
Use the MRX003-T10 DPI inhaler only with the enclosed capsules packaged in blisters.

Do not swallow the Ontipria capsules
Ontipria.
First, read the information for the patient, and then the instructions for use, before starting to use the inhaler and each time you renew your prescription. New information may appear.
Familiarize yourself with the inhaler and Ontipria capsules:
If you are using an inhaler for the first time, you should be properly trained by your doctor before using the inhaler.
Ontipria is available in a package containing capsules in blisters and an inhaler.
Use a new inhaler provided with the medicine.
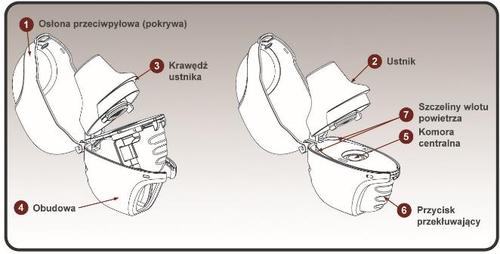
The inhaler contains:
- 1. Upper cover
- 2. Mouthpiece
- 3. Mouthpiece ridge
- 4. Housing
- 5. Central chamber
- 6. Piercing button
- 7. Air intake slots
To administer the full daily dose, perform 4 main steps
Step 1. Opening the inhaler:
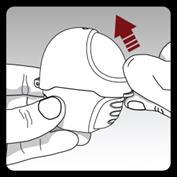
After removing the inhaler from the pouch:
Open the upper cover by lifting the front ridge.
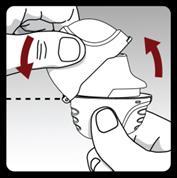
Pull the upper cover upwards to expose the mouthpiece.
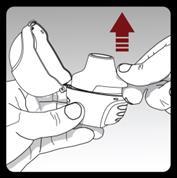
Open the mouthpiece by pulling its ridge upwards, so that the capsule chamber is visible.
Step 2. Placing the capsule in the inhaler:
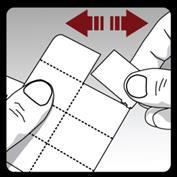
Each day, separate only 1 strip from the blister, breaking it along the perforation.
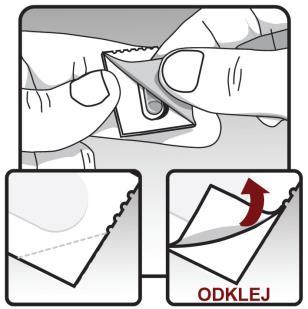
Remove the Ontipria capsule from the blister:
Do not cut the blister or use sharp objects to remove the capsule from the blister.
Lift one corner of the blister, according to the arrow on the drawing, and peel off the aluminum foil layer. Peel off the entire printed foil until the entire capsule is exposed. If you have opened more than 1 blister, do not use the additional capsule and discard it.
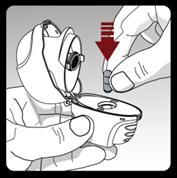
Insert the capsule into the capsule chamber in the inhaler.
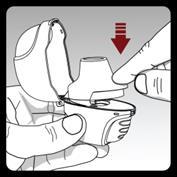
Close the mouthpiece firmly until you hear a click. Leave the upper cover open.
Step 3. Piercing the capsule
Hold the inhaler with the mouthpiece upwards.
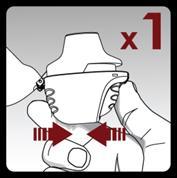
Press the piercing button until it stops and then release it. This will pierce the capsule, allowing the medicine to be administered during inhalation.
Do notpress the piercing button more than once.
Do notshake the inhaler.
Piercing the capsule may crush the capsule cap and body into small particles. Some of these particles may pass through the inhaler outlet into the mouth or throat during inhalation of the medicine. This is a normal phenomenon. The small capsule particles should not be harmful to the patient.
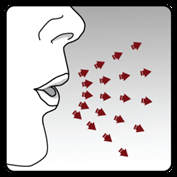
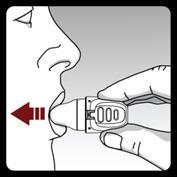
Step 4. Administering the full daily dose (2 inhalations from the same capsule):
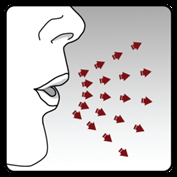
Perform a full exhalation on 1 breath,
emptying the lungs of air.
Important:Do notexhale into the inhaler.
Inhale the medicine with the next breath:
Keep your head in an upright position, looking straight ahead.
Place the inhaler in your mouth in a horizontal position.
Do not cover the air intake slots.
Close your lips tightly around the mouthpiece.
Take a slow and deep breath, so that you feel your lungs are full. You will hear and/or feel the capsule vibrating.
Hold your breath for a few seconds and remove the inhaler from your mouth at the same time.
Breathe normally.

To administer the full daily dose, completely exhale from the lungs and repeat the above steps once more. Place the inhaler in your mouth before inhaling the powder from the same capsule again.
Important:Do notpress the piercing button a second time.
Remember: To administer the full daily dose each day, you should inhale the powder from 1 capsule twice. Make sure you exhale completely from the inhaler before inhaling.
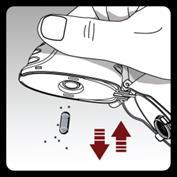
Cleaning and storing the inhaler:
After administering the full daily dose, open the mouthpiece and discard the used capsule into the trash, without touching it.
Remove any capsule fragments or accumulated powder from the inhaler without touching them, by turning the inhaler upside down and gently but firmly tapping the housing. Then, close the mouthpiece and upper cover.
Do notstore the inhaler and Ontipria capsules (blisters) in a humid place.
Ontipria capsules should always be stored in tightly closed blisters.
The inhaler should be cleanedonce a month.
Open the cap and mouthpiece.
Open the base by pushing the piercing button upwards.
Check if the capsule chamber is not contaminated with capsule particles and powder. If it is, shake them out.
Rinse the inhaler with warm water, pressing the button several times, so that the capsule chamber and piercing needle are under running water. Check if the accumulated powder and all capsule fragments have been removed.
Shake off excess water from the inhaler and dry it on a paper towel. Then, dry the inhaler in the air, leaving the upper cover, mouthpiece, and housing open until completely dry.
Do not usea hair dryer to dry the inhaler.
Do not usethe inhaler when it is wet. If necessary, you can clean the mouthpiece from the outside with a clean, damp cloth.
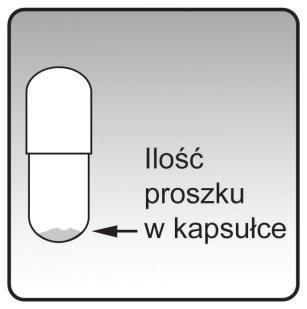
Each Ontipria capsule contains only a small amount of powder. This is one full dose taken in two inhalations.
Do notopen the Ontipria capsule, as it may not be suitable for use.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterHelm Pharmaceuticals GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OntipriaDosage form: Powder, 18 mcgActive substance: tiotropium bromideManufacturer: Ferrer Internacional, S.A.Prescription requiredDosage form: Powder, 10 mcgActive substance: tiotropium bromideManufacturer: Actavis Ltd. Laboratorios Liconsa S.A. Teva Operations Poland Sp. z o.o. Teva Pharma B.V.Prescription requiredDosage form: Powder, 18 mcg/measured doseActive substance: tiotropium bromidePrescription required
Alternatives to Ontipria in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ontipria in Ukraine
Alternative to Ontipria in Espanha
Online doctors for Ontipria
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ontipria – subject to medical assessment and local rules.














